Изменение энтальпии, отвечающее образованию одного моля вещества из простых веществ взятых в той модификации и в том агрегатном состоянии, которые отвечают наиболее их устойчивому состоянию при данной температуре и стандартном давлении называют теплотой (энтальпией) образования. Теплоты образования принято обозначать ΔfН0(т), где индекс f (от английского - formation) означает, что речь идет о теплоте образования.
Для удобства сопоставления теплоты образования относят к температуре 298,15 К (250С). Стандартные теплоты (энтальпии) образования ΔfН0298 можно найти в справочных таблицах термодинамических величин. Они измеряются в кДж/моль. Теплоты образования простых веществ в термодинамически устойчивом состоянии (Cl2, H2, O2, S – ромбическая) при стандартных условиях принимаются равными нулю.
Стандартной энтальпией сгорания вещества (ΔсН0298) (от англ. сobustion - горение) называется изменение энтальпии ΔrН0298 в реакции окисления одного моля вещества кислородом с образованием соответствующих высших оксидов в стандартных условиях. Обычно эта величина применяется по отношению к органическим соединениям, поскольку многие из неорганических веществ негорючие. Для углеводородов продуктами сгорания являются СО2(г), Н2О(ж). Азот, входящий в состав сжигающего соединения, переходит в N2(г), галогены - Cl, Br, I, - в HCl(г),, HBr(г), HI(г), сера – в SO2(г). Стандартные энтальпии сгорания определены для многих веществ и приведены в справочнике физико-химических величин.
Закон Гесса и следствия из него
В 1840 году Г.И. Гесс экспериментально установил основной закон термохимии, который является частным случаем первого закона термодинамики применительно к химическим реакциям, протекающим в изохорных или изобатных условиях.
Закон Гесса устанавливает: если из данных исходных веществ можно получить заданные конечные вещества различными путями, то суммарная теплота на одном каком-нибудь пути равна суммарной теплоте процесса на любом другом пути, т.е. тепловой эффект химических реакций зависит только от начального вида и состояния исходных веществ и продуктов реакции, но не зависит от пути процесса (от способа перехода от исходного состояния к конечному).
Он подтверждает, что при V,T = const,
Qv = ΔrU0298, а при Р,Т = const,
Qр = ΔrН0298
Проиллюстрируем закон простой схемой
Процесс окисления аммиака до оксида азота может быть осуществлен в одну стадию:
4NH3(г) + 5O2(г) = 4NO(г) + 6H2O(ж); ΔrН0298 = ΔrН1 = - 1166 кДж
Эту реакцию можно провести в две стадии: окислить аммиак кислородом с образованием газообразного азота и жидкой воды, а затем азот окислить до монооксида азота:
4NH3(г) + 3O2(г) = 2N2(г) + 6H2O(ж); ΔrН0298 = ΔrН2 = - 1531,2 кДж
2N2(г) + 2O2(г) = 4NO(г) ; ΔrН0298 = ΔrН3 = 365,2 кДж
ΔrН1 = ΔrН2 + ΔrН3
-1166 = -1531,2 + 365,2 кДж
Большое практическое значение для расчета тепловых эффектов реакций имеют следствия из закона Гесса.
Первое следствие. Тепловой эффект реакции равен разности между суммой теплот образования продуктов реакции и суммой теплот образования исходных веществ с учетом стехиометрических коэффициентов.
В качестве примера рассмотрим уравнение реакции записанной в общем виде:
v 1 А1 + v 2 А2 = v 3 А3 + v 4 А4; ΔrН0298
ΔrН0298 = [ v 3 ΔfН0298 (А3) + v 4 ΔfН0298 (А4) ] - [ v 1 ΔfН0298 (А1) + v 2 ΔfН0298 (А2)]
Второе следствие. Тепловой эффект реакции равен разности между суммой теплот сгорания исходных веществ и суммой теплот сгорания продуктов реакции с учетом стехиометрических коэффициентов.
Таким образом, для реакции:
v 1 А + v 2 В = v 3 С + v 4 D; ΔrН0298
ΔrН0298 = [ v 1 ΔсН0298 (А) + v 2 ΔсН0298 (В) ] - [ v 3 ΔсН0298 (С) + v 4 ΔсН0298 (D)]
Зависимость теплового эффекта от температуры.
Уравнение Кирхгофа.
Согласно закону Гесса можно вычислить тепловой эффект реакции при той температуре (обычно 298 К), при которой известны теплоты образования или сгорания всех реагентов. Часто бывает необходимо знать тепловой эффект реакции при различных температурах.
Запишем термодинамическое уравнение в виде:
v 1 А1 + v 2 А2 = v 3 А3 + v 4 А4; ΔrН0(т)
Обозначив энтальпию образования i –того компонента, отнесенную к 1 молю через Нi, энтальпию реакции представим в виде
ΔrН0(т) = (v 3 Н3 + v 4 Н4) - (v 1 Н1 + v 2 Н2)
Продифференцируем данное уравнение по температуре при постоянном давлении:
 (1.37)
(1.37)
Как следует из уравнения (1.30), производная 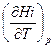 является истинной теплоемкостью i – того реагента. Из уравнения (1.37) получим
является истинной теплоемкостью i – того реагента. Из уравнения (1.37) получим
 [ΔrН0(т)]
[ΔrН0(т)]  (1.38)
(1.38)
или
 [ΔrН0(т)]
[ΔrН0(т)]  = ΔСр0 (1.38а)
= ΔСр0 (1.38а)
Частную производную от теплового эффекта по температуре можно заменить на полную:
 (ΔrН0(т))р = ΔСр0 (1.39)
(ΔrН0(т))р = ΔСр0 (1.39)
Или
d(ΔrН0(т)) = ΔСр0 · dТ (1.40)
Выражения (1.39) и (1.40) называют уравнениями Кирхгофа в дифференциальном виде. Для практического применения интегрируют (1.40), используют неопределенный интеграл
ΔrН0(т) = ΔН I +  (1.41)
(1.41)
Где ΔН I - постоянная интегрирования или интегрируют в пределах от Т1 до Т2.
В качестве нижнего предела интегрирования обычно выбирают Т1 = 298 К, так как ΔrН0298 легко вычислить по следствиям из закона Гесса
ΔrН0(т  ) = ΔrН0298 +
) = ΔrН0298 + 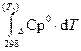 (1.42)
(1.42)
В практике используют обе интегральные формулы. Формулы Кирхгоффа применяют в трех приближениях. В первом наиболее грубом полагают ΔСр = 0, т.е. считают равными суммарные теплоемкости продуктов и исходных веществ.
Тогда ΔrН0(т  ) = ΔrН0(т
) = ΔrН0(т  ) = const, т.е. тепловой эффект считают независящим от температуры. Во втором, более точном приближении считают разность теплоемкостей постоянной величиной ΔСр0 = const; тогда ΔСр0 можно вывести за знак интеграла и для уравнения (1.42) получим следующую формулу второго приближения.
) = const, т.е. тепловой эффект считают независящим от температуры. Во втором, более точном приближении считают разность теплоемкостей постоянной величиной ΔСр0 = const; тогда ΔСр0 можно вывести за знак интеграла и для уравнения (1.42) получим следующую формулу второго приближения.
ΔrН0(т  ) = ΔrН0298 + ΔСр (Т2 - 298)
) = ΔrН0298 + ΔСр (Т2 - 298)
В третьем, наиболее точном, приближении использования формулы Кирхгоффа учитывают зависимость ΔСр от температуры в виде степенного ряда Ср0 = а + вТ. Зависимость ΔСр0 = f (T) в общем виде записывается следующим образом: ΔСр0 = Δа + ΔвТ, где Δа = Σ v iai (продуктов реакции) - Σ v jaj (исходных веществ)
Аналогично для Δв. При использовании формулы (1.42) при расчете в третьем приближении получим:
ΔrН0(т  ) = ΔrН0298 +
) = ΔrН0298 + 
После интегрирования будем иметь:
ΔrН0(т  ) = ΔrН0298 + Δа (Т2 - 298) +
) = ΔrН0298 + Δа (Т2 - 298) +  (Т22 – 2982)
(Т22 – 2982)
Рассмотрим примеры решения задач, основанных на первом и втором следствиях из закона Гесса.
Пример № 1.
Рассчитайте стандартную энтальпию (стандартный)тепловой эффект при постоянном давлении реакции
СН4(г) + 2О2(г) → СО2(г) + 2Н2О(ж)
Используя стандартные энтальпии образования исходных веществ и продуктов реакции.
Решение: По первому следствию из закона Гесса.
ΔгН0298 =  -
-  =
=
= -393,5 + 2(- 285,8) - (-74,9) - 0 = -890,2 кДж < 0
Пример № 2.
По термохимическому уравнению рассчитайте стандартную энтальпию образования исходного вещества
2КС1О3(кр) → КС1(кр) + 3О2(г); ΔгН0298 = -75,4 кДж.
Решение: По первому следствию из закона Гесса.
ΔгН0298 = 2 
 ,
,
Следовательно,
 0,5 (2
0,5 (2 
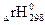 ,) = 0,5 [(2(-436,7) + 0 – (-75,4)] =
,) = 0,5 [(2(-436,7) + 0 – (-75,4)] =
= 0,5 (-873,4 + 75,4) = -399 кДж/моль
Пример № 3.
Для реакции 2NO(г) + О2(г) → 2NO2(г) рассчитайте стандартные тепловые эффекты при постоянном давлении (Qр = ΔгН0298) и объеме (Qv = ΔгU0298)
Решение:
Стандартный тепловой эффект при постоянном давлении найдем по первому следствию из закона Гесса
Qр = ΔгН0298 =  –
–  2 * 34,2 – 2 * 91,3 - 0 = -114,2 кДж =
2 * 34,2 – 2 * 91,3 - 0 = -114,2 кДж =
= - 114200Дм
По уравнению ΔгН0298 = ΔгU0298 + Δ V гRT
Qv = ΔгU0298 = ΔгН0298 - Δ V гRT
Для данной реакции Δ V г = 2 – (2+1) = - 1 моль
Стандартный тепловой эффект при постоянном объеме
Qv = ΔгU0298 = - 114200 – (-1) * 8,31 * 298 = - 111724 Дж = - 111,7 кДж
Пример № 4.
Стандартные энтальпии сгорания ацетилена С2Н2(г) и бензола С6Н6(г) соответственно равны – 1300 и – 3302 кДж /моль. Рассчитайте стандартную энтальпию реакции циклизации ацетилена.
3С2Н2(г) → С6Н6(г)
Решение: По второму следствию из закона Гесса
ΔгН0298 =  = 3 (- 1300) – (- 3302) = - 598 кДж
= 3 (- 1300) – (- 3302) = - 598 кДж






