Растворение твердых электролитов * прекращается, когда образуется насыщенный раствор, в котором устанавливается гетерогенное равновесие между твердой фазой и перешедшими в раствор ионами. Например:
CaSO4 (т)  Ca2+(р-р) + SO42–(р-р)
Ca2+(р-р) + SO42–(р-р)
В выражение константы этого гетерогенного равновесия не входит концентрация твердой фазы (см. особенности закона действия масс для гетерогенных процессов):
K= [Ca2+][SO42–]
В насыщенном растворе твердого электролита произведение концентраций его ионов есть величина постоянная при данной температуре. Она называется произведением растворимости.
ПР(CaSO4) = [Ca2+][SO42–]
Если молекула электролита содержит несколько одинаковых ионов, то концентрации этих ионов, согласно закону действия масс *, должны быть возведены в соответствующие степени. Например:
PbI2  Pb2+ + 2 I–
Pb2+ + 2 I–
ПР(PbI2) = [Pb2+][I–]2
Зная произведения растворимости, можно решать вопросы, связанные с образованием или растворением осадков при химических реакциях. Например, пусть диссоциация соли АВ происходит на два иона:
АВ  А+ + В–
А+ + В–
Обозначив растворимость через s (моль/л), получим [A+]=[B–]= s, ПР=[A+][B–]= s 2. На практике чаще возникает обратная задача определения растворимости. Для соли, диссоциирующей на два иона, 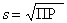 . Значения ПР можно найти в химических справочниках. Например, ПР(AgCl)=1,8·10–10, ПР(AgBr)=6·10–13, ПР(BaSO4)=1,1·10–10, ПР(HgS)=10–52. Если соль имеет общую формулу AB2, то она диссоциирует по уравнению:
. Значения ПР можно найти в химических справочниках. Например, ПР(AgCl)=1,8·10–10, ПР(AgBr)=6·10–13, ПР(BaSO4)=1,1·10–10, ПР(HgS)=10–52. Если соль имеет общую формулу AB2, то она диссоциирует по уравнению:
AB2  A2+ + 2 B–
A2+ + 2 B–
Вэтомслучае [A2+]= s, [B–]=2 s, ПР=[A2+][B–]2= s ·(2 s)2=4 s 3, 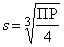 .
.
Если фактическое произведение концентраций (ПС) ионов в некотором растворе превышает значение произведения растворимости, т.е. ПС>ПР, то раствор является пересыщенным *, и из него выпадает осадок. Условие растворения осадка (ненасыщенности раствора): ПС<ПР. Оба процесса идут с одинаковой скоростью, и система приходит в состояние равновесия при ПС=ПР (насыщенный раствор).
Водородный показатель
Чистая вода обладает незначительной электрической проводимостью, которая объясняется небольшой диссоциацией воды на ионы водорода и гидроксид-ионы:
H2O  H+ + OH–
H+ + OH–
Такой процесс называется автопротолизом (самодиссоциацией). По величине электропроводности чистой воды можно вычислить концентрации ионов H+ и OH–. При 25С они равны по 10–7 моль/л.
Выражение для константы диссоциации * воды имеет вид:
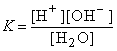 ,
,
откуда [H+][OH–]= K [H2O]= Kw.
В воде и разбавленных водных растворах концентрацию воды можно считать постоянной: [H2O]=55,5 моль/лЭта величина получается как масса одного литра воды (1000 г/л), деленная на молярную массу воды (18 г/моль)., поэтому Kw – константа. Выражение, полученное для Kw, показывает, что в воде и разбавленных водных растворах при постоянной температуре произведение концентраций ионов водорода и гидроксид-ионов есть величина постоянная. Она называется ионным произведением воды. При 250С Kw =10–14.
В кислых растворах больше концентрация ионов водорода, в щелочных – концентрация ионов OH–. Однако произведение этих молярных концентраций всегда остается постоянным. Если, например, к чистой воде добавить столько кислоты, чтобы концентрация ионов водорода повысилась до 10–3 моль/л, то концентрация гидроксид-ионов станет равной 10–11 моль/л. Следовательно, если известна величина [H+], то однозначно определяется величина [OH–]. Поэтому степень кислотности или щелочности раствора можно количественно охарактеризовать концентрацией ионов водорода:
Нейтральный раствор [H+]=10–7 моль/л;
кислый раствор [H+]>10–7 моль/л;
щелочной раствор [H+]<10–7 моль/л.
Наиболее часто используют не концентрацию [H+], а ее десятичный логарифм, взятый с обратным знаком:
pH= –lg [H+]
Эта величина называется водородным показателем. Например, если [H+]=10–5 моль/л, то pH=5; если [H+]=10–9 моль/л, то pH=9. Отсюда следует, что в нейтральном растворе pH=7, в кислом растворе pH<7, в щелочном растворе pH>7. Иногда пользуются значением гидроксидного показателя pOH= –lg[OH–]. При 25С выполняется равенство: pH+pOH=14.
Для многих процессов величина pH очень важна (для жизнедеятельности растений и животных – pH крови, почвенного раствора). Свойства природных вод, в частности их коррозионная активность, сильно зависят от pH.






