Ионное равновесие, как и любое другое, смещается при изменении концентрации одного из ионов. Например, если в раствор уксусной кислоты, диссоциирующей по уравнению
CH3COOH  H+ + CH3COO–
H+ + CH3COO–
ввести какую-либо соль этой кислоты и тем самым увеличить концентрацию ионов CH3COO–, то в соответствии с принципом Ле-Шателье * равновесие смещается влево. Отсюда следует, что введение в раствор слабого электролита * одноименных ионов (т.е. ионов, одинаковых с одним из ионов электролита) уменьшает степень диссоциации * этого электролита.
Аналогично нарушается равновесие в случае малорастворимого электролита (соли). Например, если к насыщенному раствору сульфата кальция CaSO4 добавить другой, хорошо растворимый сульфат (K2SO4), то вследствие увеличения концентрации ионов SO42– равновесие сместится в сторону образования кристаллов (образуется осадок CaSO4). Этот процесс прекратится, когда произведение концентраций [Ca2+] и [SO42–] станет равно произведению растворимости *, т.е. установится новое состояние равновесия.
На основании рассмотренных примеров можно сделать следующий вывод: реакции в растворах электролитов всегда идут в сторону образования наименее диссоциированных или наименее растворимых веществ. Из этого, в частности, следует, что сильные кислоты вытесняют слабые из растворов их солей:
CH3COONa + HCl = CH3COOH + NaCl
Суть этой реакции более точно отражается ионно-молекулярным уравнением, где формулы слабых электролитов записаны в виде молекул, а сильных – в виде ионов:
CH3COO– + Na+ + H+ + Cl– = CH3COOH + Na+ + Cl–
или в сокращенном виде. Сокращенное ионное уравнение отражает самую суть происходящего процесса. Вступают в реакцию или образуются в ней в действительности только те частицы (ионы или молекулы), которые записаны в сокращенном уравнении.:
CH3COO– + H+ = CH3COOH
Аналогично протекают реакции между сильными основаниями и солями слабых оснований. Например:
FeSO4 + 2 NaOH = Na2SO4 + Fe(OH)2
Fe2+ + SO42– + 2 Na+ + 2 OH– = SO42– + 2 Na+ + Fe(OH)2
Fe2+ + 2 OH– = Fe(OH)2
Гидролизсолей
Химическая реакция обменного характера растворяемого вещества с растворителем называется сольволизом. Если растворителем является вода, то процесс – гидролиз (частный случай сольволиза).
Суть гидролиза солей заключается в том, что происходит смещение равновесия диссоциации воды вследствие связывания одного из ее ионов с образованием малодиссоциированного или труднорастворимого продукта. Гидролиз идет по-разному в зависимости от силы кислоты и основания, образовавших соль. Рассмотрим различные случаи.
1. Соль образована слабой кислотой и сильным основанием (CH3COONa, KCN, Na2CO3).
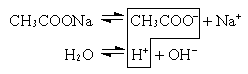
или CH3COO– + Na+ + H2O  CH3COOH + Na+ + OH–
CH3COOH + Na+ + OH–
CH3COO– + H2O  CH3COOH + OH–
CH3COOH + OH–
Так как уксусная кислота слабо диссоциирует, ацетат-ион связывает ион H+, и равновесие диссоциации воды смещается вправо согласно принципу Ле Шателье. В растворе накапливаются ионы OH– (pH>7)*.
Если соль образована многоосновной кислотой, то гидролиз идет ступенчато. Например, гидролиз карбоната:
I ступень: CO32– + H2O  HCO3– + OH–
HCO3– + OH–
II ступень: HCO3– + H2O  H2CO3 + OH–
H2CO3 + OH–
Практическое значение обычно имеет только процесс, идущий по первой ступени, которым, как правило, и ограничиваются при оценке гидролиза солей. Равновесие гидролиза по второй ступени значительно смешено влево по сравнению с равновесием первой ступени, поскольку на первой ступени образуется более слабый электролит (HCO3–), чем на второй (H2CO3)
2. Соль образована сильной кислотой и слабым основанием (NH4NO3, AlCl3, Fe2(SO4)3).
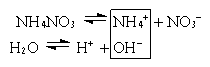
NH4+ + NO3– + H2O  NH4OH + NO3– + H+
NH4OH + NO3– + H+
NH4+ + H2O  NH4OH + H+
NH4OH + H+
(pH<7)
В случае многозарядного катиона гидролиз протекает ступенчато, например:
I ступень: Cu2+ + HOH  CuOH+ + H+
CuOH+ + H+
II ступень: CuOH+ + HOH  Cu(OH)2 + H+
Cu(OH)2 + H+
При этом концентрация ионов водорода и pH среды * в растворе также определяются главным образом первой ступенью гидролиза.
3. Соль образована слабой кислотой и слабым основанием (CH3COONH4, (NH4)2CO3).
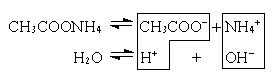
CH3COO– + NH4+ + H2O  CH3COOH + NH4OH
CH3COOH + NH4OH
В этом случае образуются два малодиссоциированных соединения, и pH раствора зависит от относительной силы кислоты и основания.
Если продукты гидролиза могут удаляться из раствора- например, в виде осадка или газообразного вещества., то гидролиз протекает до конца. Например:
Al2S3 + 3 H2O  Al(OH)3↓ + H2S↑
Al(OH)3↓ + H2S↑
4. Соли, образованные сильной кислотой и сильным основанием, гидролизу не подвергаются, т.к. единственным малодиссоциирующим соединением является H2O.
Взаимное усиление гидролиза. Допустим, что в разных сосудах установились равновесия:
CO32– + H2O  HCO3– + OH–
HCO3– + OH–
Al3+ + H2O  AlOH2+ + H+
AlOH2+ + H+
Обе соли гидролизованы незначительно, но если растворы смешать, то происходит связывание ионов H+ и OH–. В соответствии с принципом Ле-Шателье * оба равновесия смещаются вправо, и гидролиз протекает полностью:
2 AlCl3 + 3 Na2CO3 + 3 H2O = 2 Al(OH)3 + 3 CO2 + 6 NaCl
Это называется взаимным усилением гидролиза.
Жесткость воды и методы её устранения
В природе чистая вода не встречается: она всегда содержит примеси каких – либо веществ. Взаимодействуя с солями, содержащимися в земной коре, вода приобретает определённую жёсткость.
Жёсткость воды – с овокупность свойств, обусловленных содержанием в ней катионов кальция Са2+ и катионов магния Mg2+.
Катионы кальция Са2+ обуславливают кальциевую жёсткость, а катионы магния Mg2+ - магниевую жёсткость воды.
Общая жёсткость складывается из кальциевой и магниевой, т.е. из суммарной концентрации в воде этих катионов.
Различают карбонатную (временную) и некарбонатную (постоянную) жёсткость.
Карбонатная жёсткость вызвана присутствием гидрокарбонатов кальция и магния. При кипячении гидрокарбонаты разрушаются, а образующиеся малорастворимые карбонаты выпадают в осадок, и общая жёсткость воды уменьшается на значение карбонатной жёсткости. При кипячении катионы кальция Са2+ осаждаются в виде карбоната кальция:
Са2+ + 2НСО3− = СаСО3↓ + Н2О + СО2
Катионы магния Mg2+ осаждаются в виде основного карбоната или в виде гидроксида магния (при рН > 10,3):
2Mg2+ + 2НСО3− + 2OH− = (MgOH)2CO3↓ + Н2О + СО2
(гидроксид –ионы OH− образуются за счёт взаимодействия ионов НСО3− с водой: НСО3− + Н2О ↔ Н2СО3 + ОН−).
Некарбонатная жёсткость определяется содержанием в воде кальциевых и магниевых солей сильных кислот, главным образом сульфатов и хлоридов. При кипячении эти соли не удаляются.
По величине жёсткости воду делят на 6 классов.
Таблица 1.3.1.
| Классы | Жёсткость воды (ммоль экв/л) |
| 1 (очень мягкая) 2 (мягкая) 3 (средней жёсткости) 4 (довольно жёсткая) 5 (жёсткая) 6 (очень жёсткая) | 0 – 1,5 1,5 – 3,0 3,0 – 4,5 4,5 – 6,0 6,0 – 10,0 свыше 10,0 |
Жёсткая вода оказывает вредное действие на технологические процессы и вызывает неприятные явления при использовании её в быту.
Обработка воды, приводящая к снижению жесткости воды, называется умягчением воды.
Существующие способы умягчения можно разделить на 3 группы:
1. реагентные (химические) методы умягчения воды. Химическая обработка воды основана на переводе растворимых солей кальция и магния в труднорастворимыее соли; В качестве реагентов–осадителей применяют известь, соду, едкий натр, фосфаты натрия и др. Чаще применяется сода в смеси с известью или едким натром;
2. умягчение воды методом ионного обмена (пропускание воды через ионообменные смолы);
3. термическое умягчение воды ( кипячение).
Для устранения временной жёсткости воды применяют:
– термическое умягчение (кипячение):
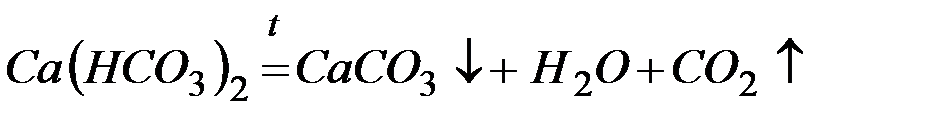
– реагентное умягчение гашёной известью:
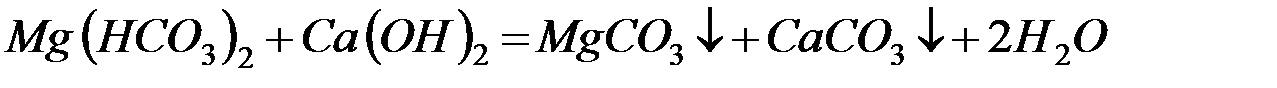
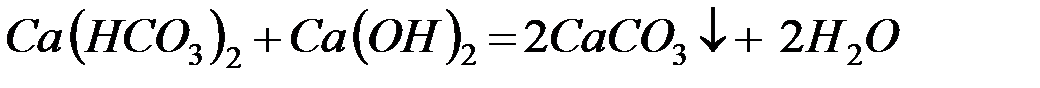
Известь нейтрализует углекислоту, которая является одной из причин коррозии металлов, осаждает железо и способствует коагуляции коллоидов, например, кремниевой кислоты.
Для устранения общей жёсткости методом осаждения используют:
– карбонат натрия (соду):
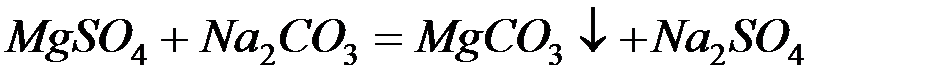
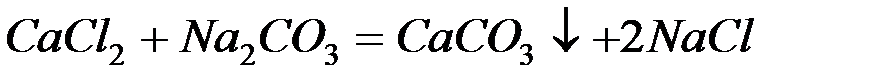
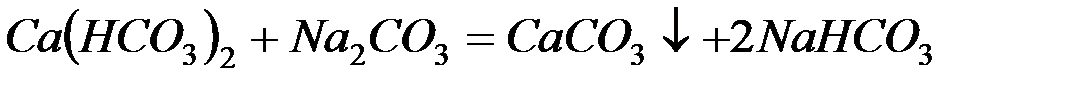
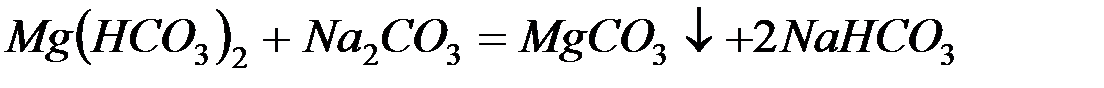
– фосфат натрия (тринатрийфосфат):
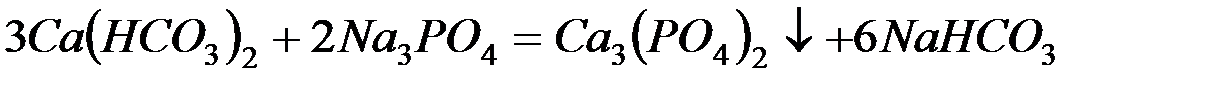
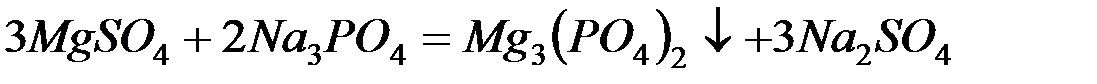
В последнее время для очистки воды стали широко применять иониты (ионообменные смолы). Метод ионного обмена – катионирование основан на фильтровании через слой катионита, при котором происходит замещение ионов Ca2 и Mg2+, содержащихся в воде, на катионы Na+, K+ или NH+4, содержащиеся в твёрдой фазе катионита. В качестве катионитов в основном применяют иониты КУ-I и КУ-2.
В некоторых случаях требуется удалить из воды не только катионы Ca2+ и Mg2+, но и другие катионы и анионы. В таких случаях воду пропускают последовательно через катионит, содержащий в обменной форме водородные ионы (Н+-катионит) и анионит, содержащий гидроксильные ионы (ОН––анионит). В итоге вода освобождается как от катионов, так и от анионов солей. Такая обработка воды называется обессоливанием:
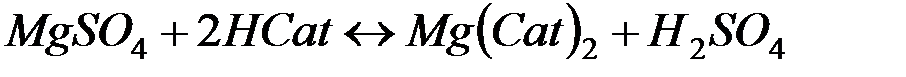
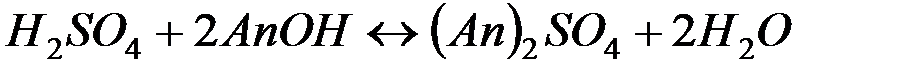
Обменная ёмкость 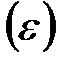 катионита определяется из соотношения:
катионита определяется из соотношения:
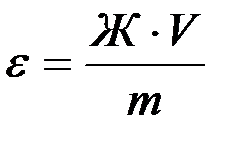 (ммоль экв/г),
(ммоль экв/г),
где: V- объём воды, пропущенный через катионит, л;
m – масса катионита, г.
Общая жёсткость воды может быть рассчитана по формуле:
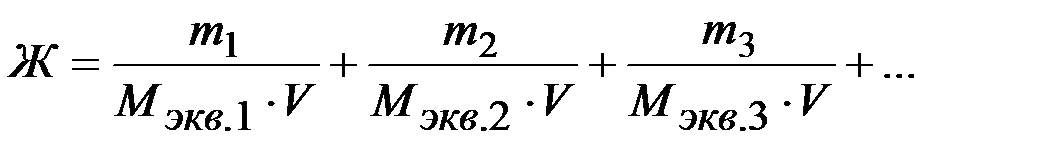 (ммоль экв/л)
(ммоль экв/л)
где: m1, m2, m3 – массы содержащихся в воде катионов кальция, магния (или соответствующих им солей), мг;
Мэкв.1 , Мэкв.2 , Мэкв.3 – молярные массы эквивалентов металлов (или соответствующих им солей), г/моль экв;
V– объём воды, л.
Для расчётов можно также применять формулу, которая выражается суммой миллиэквивалентов (мэкв) катионов кальция и магния, содержащихся в 1 л воды (моль/л). Один миллимоль жёсткости отвечает содержанию 20,04 мг/л катионов кальция Са2+ или 12,16 мг/л катионов магния Mg2+, то согласно определению, общую жёсткость воды Ж (в ммоль/л) можно вычислить по формуле: 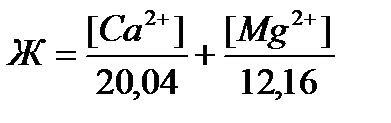 , где [Са2+] и [Mg2+] – концентрация соответствующих ионов в мг/л.
, где [Са2+] и [Mg2+] – концентрация соответствующих ионов в мг/л.
Вопросы для самоконтроля
1. Почему растворы кислот, оснований и солей не подчиняются законам идеальных растворов? Что такое изотонический коэффициент?
2. Что называется электролитом? Чем отличаются сильные электролиты от слабых?
3. В чём заключается механизм электролитической диссоциации? Одинаков ли он для соединений с ионным и ковалентным полярным типом химической связи?
4. Что называется степенью диссоциации электролита? От чего она зависит? Как она связана с константой диссоциации?
5. Что такое константа кислотности и константа основности?
6. Дать понятие «активность», «коэффициент активности» и «ионная сила» раствора.
7. Что такое ионное произведение воды? Что такое рН?
8. Какие растворы называются буферными? Как вычислить рН буферного раствора?
9. Что называется реакцией гидролиза? Какие типы гидролиза существуют? Каковы математические выражения константы гидролиза и степени гидролиза?
10. Что называется произведением растворимости? Какие условия выпадения и растворения осадка малорастворимого электролита?
11. Какие виды жёсткости воды существуют? Как рассчитать жёсткость воды?






