Приклад 1. Визначити еквівалент і фактор еквівалентності фосфатної (V) кислоти у реакції з розчином натрій гідроксиду:
H3PO4 + 3NaOH = Na3PO4 + 3H2O.
Розв’язування
Один гідроксид-іон еквівалентний одному гідроген-іону. Фактор еквівалентності визначається з того, що 3 моль NaOH реагують з 1 моль H3PO4. Отже, еквівалент H3PO4 становить  частину моля H3PO4, фактор еквівалентності дорівнює
частину моля H3PO4, фактор еквівалентності дорівнює  :
:
ƒекв (H3PO4)=  .
.
У іншій реакції (H3PO4 + 2NaOH = Na2НPO4 + 2H2O) 2 моль NaOH реагують з 1 моль H3PO4, що відповідає 2 моль гідроген-іонів. Отже, еквівалент H3PO4 у цій реакції становить ½ частку моль H3PO4. Фактор еквівалентності дорівнює ½:
ƒекв (H3PO4)=½.
Приклад 2. Визначити еквівалент і фактор еквівалентності K2Cr2O7 у реакції:
K2Cr2O7 + 6КI + 7H2SO4 = Cr2(SO4)3 + 4K2SO4 +3I2 + 7H2O.
Розв’язування
Це реакція окиснення-відновлення. Еквівалент визначається кількістю електронів, що приєднує чи віддає частинка в реакції:
 + 6 ē + 14Н+ = 2Cr3+ + 7H2O.
+ 6 ē + 14Н+ = 2Cr3+ + 7H2O.
Один йон  приєднує 6 електронів. Отже, еквівалент K2Cr2O7 у цій реакції становить
приєднує 6 електронів. Отже, еквівалент K2Cr2O7 у цій реакції становить  частку моля K2Cr2O7, а фактор еквівалентності дорівнює
частку моля K2Cr2O7, а фактор еквівалентності дорівнює  :
:
ƒекв (K2Cr2O7)=  .
.
Приклад 3. На титрування розчину щавлевої кислоти розчином натрій гідроксиду з молярною концентрацією NaOH 0,1235 моль/дм 3 було витрачено 13,50 см 3 розчину NaOH. Речовини реагували між собою згідно з рівнянням реакції:
H2С2O4 + 2NaOH = Na2С2O4 + 2H2O.
Знайти вміст щавлевої кислоти у розчині.
Дано:
c (NaOH)=0,1235 моль/дм 3 М (H2С2O4)=90 г/моль
 V (NaOH(р))=13,50 см 3
V (NaOH(р))=13,50 см 3
m (H2С2O4) –?
Розв’язування
У даній реакції фактор еквівалентності H2С2O4 дорівнює ½


Приклад 4. Розрахувати масу натрій гідроксиду, що міститься у 500,00 см 3 розчину якщо на титрування 20,00 см 3 цього розчину треба затратити 20,80 см 3 розчину хлоридної кислоти, титр якого дорівнює 0,002022 г/см 3.
Дано:
V 1(NaOH(р))=500,00 см 3 M ( NaOH)=39,99 г/моль
NaOH)=39,99 г/моль
V 2(NaOH(р))=20,00 см 3 M ( HCl)=36,46 г/моль
HCl)=36,46 г/моль
V (HCl(р))=20,80 см 3
Т (HCl)=0,002022 г/см 3
 m (NaOH) –?
m (NaOH) –?
Розв’язування


Приклад 5. Розрахувати масу оцтової кислоти, що знаходиться у розчині об’ємом 500,00 см 3, ящо на титрування 25,00 см 3 цього розчину витрачається 20,50 см 3 розчину натрій гідроксиду з молярною концентрацією еквівалента речовини NaOH 0,1145 моль/дм 3.
Дано:
V 1(CH3COOH(р))=500,00 см 3 М ( CH3COOH)=60,05 г/моль
CH3COOH)=60,05 г/моль
V 2(CH3COOH(р))=25,00 см 3 М ( NaOH)=39,99 г/моль
NaOH)=39,99 г/моль
V (NaOH(р))=20,50 см 3
 c (
c ( NaOH)=0,1145 моль/дм 3
NaOH)=0,1145 моль/дм 3
m (CH3COOH) –?
Розв’язування
Знаходять титр розчину NaOH з молярною концентрацією еквівалента речовини NaOH 0,1145 моль/дм 3 за оцтовою кислотою:


Далі визначається маса оцтової кислоти у загальному об’ємі розчину:

= 
Приклад 6. Титр розчину арґентум нітрату дорівнює 0,01702 г/см 3. Визначити титр цього розчину за натрій хлоридом та молярну концентрацію еквівалента розчину AgNO3.
Дано:
 Т (AgNO3)=0,01702 г/см 3 М (
Т (AgNO3)=0,01702 г/см 3 М ( AgNO3)=169,9 г/моль
AgNO3)=169,9 г/моль
с ( AgNO3) –? М (
AgNO3) –? М ( NaСl)=58,46 г/моль
NaСl)=58,46 г/моль
Т (AgNO3/NaСl) –?
Розв’язування


Приклад 7. На титрування суміші натрій карбонату і калій карбонату загальною масою 0,4000 г було використано 22,00 см 3 розчину хлоридної кислоти з молярною концентрацією еквівалента речовини НСl 0,3000 моль/дм 3. Розрахувати масові частки (%) Na2СO3 і К2СO3 у суміші.
Дано:
m (Na2СO3)+ m (К2СO3)=0,4000 г М (1/2Na2СO3)=52,994 г/моль
V (НСl(р))=22,00 см 3 М (1/2К2СO)=69,103 г/моль
 с (
с ( НСl)=0,3000 моль/дм 3
НСl)=0,3000 моль/дм 3
w (Na2СO3) –?
w (К2СO3) –?
Розв’язування
Якщо w – масова частка (%) Na2СO3 у суміші, то кількості речовини еквівалентів Na2СO3 і К2СO3, що містяться у даній масі суміші, будуть:


У точці еквівалентності
n (½Na2СO3) + n (½К2СO3) = n (НСl),
де 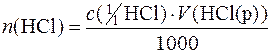 – кількість речовини НСl, яка була витрачена на титрування.
– кількість речовини НСl, яка була витрачена на титрування.
Тоді:

Підставляючи числові значення, маємо:
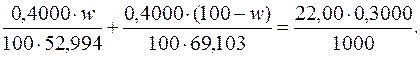
Звідси: w=46,12.
Отже, масова частка Na2СO3 у досліджуваній суміші становить 46,12%, а масова частка К2СO3 – (100–46,12)=53,88%.
Приклад 8. Проводячи аналіз стічної води, до проби цієї води об’ємом 100,0 см 3 додали при нагріванні 25,00 см 3 розчину барій хлориду з молярною концентрацією речовини ВаCl2 0,02 моль/дм 3 (K =0,9816). Надлишок ВаCl2 відтитрували у присутності амоніачного буферу, що містив магній комплексонат і еріохром чорний Т, витративши при цьому 17,00 см 3 розчину ЕДТА з молярною концентрацією ЕДТА 0,02 моль/дм 3 (K =1,018). Розрахувати концентрацію йонів  у стічній воді в г/дм 3.
у стічній воді в г/дм 3.
(K – поправочний коефіцієнт до концентрацій).
Дано:
V (стічної води)=100,0 см 3 М ( )=96,06 г/моль
)=96,06 г/моль
V (ВаCl2(р))=25,00 см 3
с (ВаCl2)=0,02 моль/дм 3
K 1(ВаCl2(р))=0,9816
V (ЕДТА(р))=17,00 см 3
с (ЕДТА)=0,02 моль/дм 3
K 2(ЕДТА(р))=1,018
 с (
с ( ) в г/дм 3 –?
) в г/дм 3 –?
Розв’язування
У точці еквівалентності справедливе співвідношення:
n ( ) = n (ВаCl2) – n (ЕДТА).
) = n (ВаCl2) – n (ЕДТА).
Якщо позначити концентрацію йонів  у стічній воді через х г/дм 3, тоді:
у стічній воді через х г/дм 3, тоді:


Підставляючи числові значення, отримуємо:

Отже, концентрація йонів  у стічній воді становить 0,1390 г/дм 3.
у стічній воді становить 0,1390 г/дм 3.
Приклад 9. У мірній колбі об’ємом 500,00 см 3 розчинили 5,360 г технічного калій хлориду. До 25,00 см 3 отриманого розчину додали 50,00 см 3 розчину аргентум нітрату з молярною концентрацією речовини AgNO3 0,1 моль/дм 3 (K =0,8470). На титрування надлишку AgNO3 витрачається 23,88 см 3 розчину амоній тіоціанату (Т (NH4SCN/Ag+)=0,01063 г/см 3). Розрахувати масову частку (%) КCl у зразку.
Дано:
V 1(КCl(р))=500,0 см 3 М (КCl)=74,55 г/моль
V 2(КCl(р))=25,00 см 3 М (Ag+)=107,878 г/моль
m (КCl)=5,360 г
V (AgNO3(р))=50,00 см 3
с (AgNO3)=0,1 моль/дм 3
K =0,8470
V (NH4SCN(р))=23,88 см 3
Т (NH4SCN/Ag+)=0,01063 г/см 3.
 w (КCl) –?
w (КCl) –?
Розв’язування
Кількість речовини КCl визначається із співвідношення:


Масову частку (%) КCl у зразку визначають за формулою:

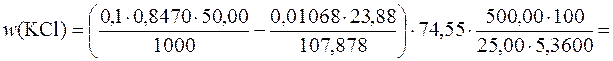

Приклад 10. Чому дорівнює індикаторна похибка титрування розчину хлоридної кислоти (с ( НСl)=0,1 моль/дм 3) розчином натрій гідроксиду (с (
НСl)=0,1 моль/дм 3) розчином натрій гідроксиду (с ( NaOH)=0,1 моль/дм 3) з індикатором метиловим червоним (рТ =5)?
NaOH)=0,1 моль/дм 3) з індикатором метиловим червоним (рТ =5)?
Розв’язування
Оскільки титрування закінчиться при p H = 5, то наявність у розчині сильної кислоти, так як розчин недотитрований, викличе Н+-похибку (зі знаком мінус).
Н+-похибка = 
де V 1 – об’єм кислоти, яка титрується;
V 2 – загальний об’єм розчину в кінці титрування.
Так як у процесі титрування використовувались розчини однакової концентрації, то V 2=2 V 1.
Тоді Н+-похибка = 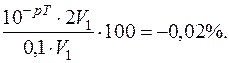
Похибка мала, тому для даного титрування можна застосувати індикатор метиловий червоний.
Приклад 11. Чи можна відтитрувати розчин натрій гідроксиду (с ( NaOH)=0,02 моль/дм 3) розчином хлоридної кислоти (с (
NaOH)=0,02 моль/дм 3) розчином хлоридної кислоти (с ( НCl)=0,02 моль/дм 3): а) з фенолфталеїном (рТ =9); б) з метиловим оранжевим (рТ =4)?
НCl)=0,02 моль/дм 3): а) з фенолфталеїном (рТ =9); б) з метиловим оранжевим (рТ =4)?
Розв’язування
У першому випадку точка еквівалентності досягається при p H=7. Після закінчення титрування (p H=9) у розчині ще є невідтитрований луг, що викличе ОН–-похибку.
ОН–-похибка = 
ОН–-похибка = 
Похибка незначна, отже фенолфталеїн цілком придатний для визначення кінця титрування у даному випадку.
У другому випадку титрування закінчується за наявності у розчині надлишку сильної кислоти (p H=4), тобто буде мати місце Н+-похибка зі знаком плюс, так як розчин перетитрований:
Н+-похибка = 
Через велику похибку індикатор метиловий оранжевий у даному випадку титрування застосувати не можна.
Приклад 12. Мідний купорос CuSO4·5H2O масою 6,2508 г розчинили у мірній колбі на 250 см 3 і довели водою до риски. До 25,00 см 3 отриманого розчину додали надлишок КІ. На титрування йоду, що виділився, було витрачено 24,1 см 3 розчину натрій тіосульфату з молярною концентрацією еквівалента Na2S2O3 0,1015 моль/дм 3. Розрахувати масову частку йонів Cu2+ у мідному купоросі.
Дано:
m (CuSO4)=6,2508 г M (Cu2+)=63,54 г/моль
V 1(CuSO4(p))=250 см 3
V 2(CuSO4(p))=25,00 см 3
V (Na2S2O3(p))=24,1 см 3
с (½Na2S2O3)=0,1015 моль/дм 3
 w (Cu2+) –?
w (Cu2+) –?
Розв’язування
Визначають молярну концентрацію еквівалента речовини CuSО4 у розчині:






