| Дано: Уравнение реакции |
| Уравнять реакцию и указать окислитель и восстановитель. Определить направление протекания реакции. Кс -? |
РЕШЕНИЕ:
а) Для того, чтобы определить направление протекания окислительно-восстановительной реакции, надо сопоставить силу окислителей для следующих полуреакций (см. таблицу)

Cu2+ + e- = Cu+ φ  = +0,15 B
= +0,15 B
I2 + 2e- = 2I- φ  = +0,54 B
= +0,54 B
Из двух приведенных окислителей более сильным окислителем будет I2, поскольку φ  > φ
> φ  .
.
I2 находится в продуктах реакции, следовательно, реакция будет протекать справа налево т.е. реально протекающей будет реакция:
CuCl + KCl + I2 <=> KI + CuCl2
Расставляем коэффициенты методом электронного баланса:
Cu+1Cl + KCl + I20 = Cu+2Cl2 + KI-1

 НОК ДМ
НОК ДМ
Cu+1 - e- = Cu+2 2
 I
I  + 2e- = 2I-1 1
+ 2e- = 2I-1 1
I20 + 2Cu+1 = 2Cu+2 + 2I 
2CuCl + 2KCl + I2 <=> 2KI + 2CuCl2
Константа равновесия реакции определяется из уравнения
 (9.1)
(9.1)



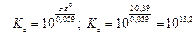 =1,66·1013. (9.2)
=1,66·1013. (9.2)
где z - число отданных или принятых электронов (НОК = 2);
F = 96500 Кл/моль; ε˚ - стандартное напряжение ОВР, В
ε˚ = φ˚ок - φ˚восст (9.3)
ε˚ =  = 0,54-0,15 = 0,39В
= 0,54-0,15 = 0,39В
Ответ: окислитель - I2; восстановитель – CuCl, приведенная в условии задачи реакция (а) протекает справа налево, КС =1,66·1013.
б) Из таблицы определяем стандартные электродные потенциалы окислителей для реакции
KI + FeCl3 <=> FeCl2 + KCl + I2
Fe3+ + e- = Fe+2 φ  = +0,77 B
= +0,77 B
I2 - 2e- = 2I- φ  = +0,54 B
= +0,54 B
Из двух приведенных окислителей более сильным окислителем будет Fe+3, поскольку φ  > φ
> φ  .
.
Fe+3 находится в исходных веществах, следовательно, реакция будет протекать слева направо т.е. реально протекающей будет реакция (б), приведенная в условии задачи:
KI + FeCl3 <=> FeCl2 + KCl + I2
Расставляем коэффициенты методом электронного баланса:
KI-1 + Fe+3Cl3 = Fe+2Cl2 + KCl + I20
НОК ДМ



 I-1 - e- = I0 1 2
I-1 - e- = I0 1 2
1 2
Fe+3 + e- = Fe+2 1 2
 I-1 + Fe+3 = Fe+2 + I0
I-1 + Fe+3 = Fe+2 + I0
Поскольку после реакции образуется четное число атомов иода, значения НОК и ДМ удваиваем.
2KI + 2FeCl3 <=> 2FeCl2 + 2KCl + I2
Константа равновесия реакции определяется из уравнения
 (9.1)
(9.1)
 =6,3·107. (9.2)
=6,3·107. (9.2)
где z - число отданных или принятых электронов (НОК = 2);
F = 96500 Кл/моль; ε˚ - стандартное напряжение ОВР, В
ε˚ = φ˚ок - φ˚восст (9.3)
ε˚ = 0,77-0,54 = 0,23В
Ответ: окислитель – FeCl3; восстановитель – KI, реакция протекает слева направо, Кс =6,3·107.
ГАЛЬВАНИЧЕСКИЕ ЭЛЕМЕНТЫ.
КОРРОЗИЯ МЕТАЛЛОВ
УРОВЕНЬ А
1. а) Алюминиевый электрод погружен в 5∙10-4М раствор сульфата алюминия. Вычислить значение потенциала алюминиевого электрода.

РЕШЕНИЕ:
Электродный потенциал алюминия рассчитываем по уравнению Нернста:
 = =  + + 
|
По таблице 11.1 определяем стандартный электродный потенциал алюминия
 = -1,67В.
= -1,67В.
Записываем уравнение электродного процесса, протекающего на поверхности алюминиевого электрода в растворе соли:
Al - 3ē = Al3+
n – число электронов, участвующих в электродном процессе.
Для данной реакции n равно заряду иона алюминия Al3+(n=3).
Рассчитываем концентрацию ионов алюминия в растворе Al2(SO4)3:
 =
=  ∙α∙
∙α∙ 
Разбавленный раствор Al2(SO4)3 – сильный электролит.
Следовательно, α = 1. По уравнению диссоциации Al2(SO4)3:
Al2(SO4)3 = 2Al3+ + 3SO 
число ионов Al3+, образующихся при диссоциации одной молекулы Al2(SO4)3 равно 2.
Следовательно,  =2
=2
Тогда  = 5∙10-4∙1∙2 =
= 5∙10-4∙1∙2 = 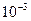 моль/л.
моль/л.
Рассчитываем электродный потенциал алюминиевого электрода:
 = -1,67 +
= -1,67 +  = -1,73В.
= -1,73В.
Ответ:  = -1,73В.
= -1,73В.






