|

| РЕШЕНИЕ:
Ж= 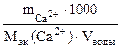 + +  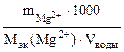
 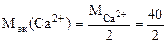 =
=20 г/моль (мг/ммоль) =
=20 г/моль (мг/ммоль)
|
 =12 г/моль (мг/ммоль).
=12 г/моль (мг/ммоль).
1000 – перевод г в мг
Ж= 
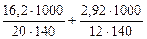 =7,53 ммоль/л.
=7,53 ммоль/л.
Ответ: Ж= 7,53 ммоль/л.
2. Определить массу сульфата кальция в 200 л воды, если жесткость, обусловленная этой солью, равна 9 ммоль/л.

| РЕШЕНИЕ:
nэк (Ca+2) = nэк (CaSO4)
Ж = 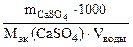
|
откуда 
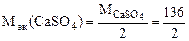 = 68 г/моль (мг/ммоль)
= 68 г/моль (мг/ммоль)
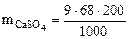 =122,4 г
=122,4 г
Ответ: 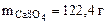
3. Постоянная жесткость воды объемом 10 л обусловлена наличием сульфата магния. Определить массу буры (Na2B4O7), необходимую для устранения постоянной жесткости, равной 5 ммоль/л. Написать уравнение протекающей реакции
|

| РЕШЕНИЕ:
MgSO4 + Na2B4O7 = MgB4O7↓ + Na2SO4
nэк(MgSO4) = nэк(Na2B4O7)
Ж = 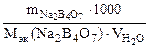
|
Mэк(Na2B4O7) = 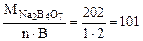 г/моль
г/моль
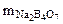 =
= 
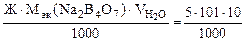 =
=
= 5,05 г
Ответ:  = 5,05 г
= 5,05 г
УРОВЕНЬ В
1. На титрование 100 см3 воды, содержащей гидрокарбонат магния, ушло 12 см3 0,15 н раствора HCl. Написать уравнение протекающей реакций. Рассчитать жесткость воды и определить массу соли, содержащейся в 40 л этой воды.

| РЕШЕНИЕ:
Составляем уравнение реакции:
Mg(HCO3)2 + 2HCl = MgCl2 +
+ 2H2O + 2CO2↑
Ж =  =
= =
=   . .
|
Согласно закону эвивалентов
nэкMg(HCO3)2 = nэк(HCl) = Cэк(HCl)·V 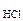
откуда
Ж = 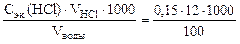 = 18 ммоль/л.
= 18 ммоль/л.
Определяем массу гидрокарбоната магния содержащейся в 40 л воды:
Ж = 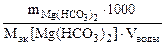 , откуда
, откуда
 =
= 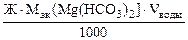 =
=
= 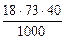 =52,56 г.
=52,56 г.
Мэк[Mg(HCO3)2] = 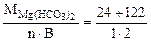 =73 г/моль (мг/ммоль);
=73 г/моль (мг/ммоль);
Ответ: Ж = 18 ммоль/л,  = 52,56 г.
= 52,56 г.
2. При кипячении 2 л воды (при рН>10,3), содержащей только гидрокарбонат магния, образовался осадок массой 28 мг. Написать уравнение протекающей реакции. Определить жесткость воды и массу соли в 0,5 м3 этой воды.
|

| РЕШЕНИЕ: При кипячении воды, содержащей гидрокарбонаты кальция, магния или железа, осадок выпадает в результате следующих реакций: |
Сa(HСО3)2 = CaCO3↓ + H2O + CO2↑
Mg(HCO3)2 = Mg(OH)2↓ + 2CO2↑ (при рН>10,3)
Fe(HCO3)2 = Fe(OH)2↓ + 2CO2
Так как все вещества реагируют и образуются в равных количествах их эквивалентов: nэк(Mg(HCO3)2) = nэк(Mg(OH)2), поэтому жесткость можно рассчитать по массе осадка гидроксида магния.
Ж = 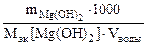 =
= 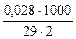 = 0,48 ммоль/л
= 0,48 ммоль/л
Мэк[Mg(OH)2] = 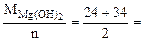 29 г/моль (мг/ммоль)
29 г/моль (мг/ммоль)
Массу гидрокарбоната магния в 0,5 м3 воды определяем по формуле.
Ж = 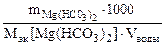
откуда  =
= 
Мэк[Mg(НСO3)2] = 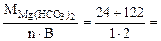 73 г/моль (мг/ммоль)
73 г/моль (мг/ммоль)
 =
=  = 17,52 г.
= 17,52 г.
Ответ: Ж = 0,48 ммоль/л,  = 17,52 г.
= 17,52 г.
3. Для устранения жесткости к 10 л природной воды, которая содержит 0,015% гидрокарбоната кальция и 0,005% гидрокарбоната магния, добавили гашеную известь. Плотность воды 1 г/см3. Написать уравнения протекающих реакций. Определить массу гашеной извести, необходимую для устранения временной жесткости.

| РЕШЕНИЕ: Гидроксид кальция Ca(OH)2 (гашеная известь) используется для устранения временной жесткости. При этом протекают следующие реакции: |
Ca(HCO3)2 + Ca(OH)2 = 2CaCO3↓ + 2H2O
Mg(HCO3)2 + 2Ca(OH)2 = Mg(OH)2↓ + 2CaCO3↓ + 2H2O
Необходимые для расчета массы солей найдем по их массовым долям в воде
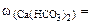
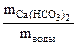 ∙100
∙100
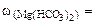
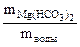 ∙100
∙100
Откуда 
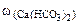 ∙10-2 ∙mводы =
∙10-2 ∙mводы = 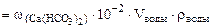 = 0,015·10-2 10·103·1 = 1,5 г.
= 0,015·10-2 10·103·1 = 1,5 г.
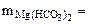
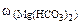 ∙10-2 ∙mводы =
∙10-2 ∙mводы = 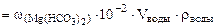 = 0,005·10-2·10·103·1 = 0,5 г.
= 0,005·10-2·10·103·1 = 0,5 г.
Ж = 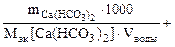
 =
=
= 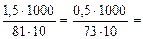 2,53 ммоль/л.
2,53 ммоль/л.
Мэк[Сa(НСO3)2] =  81 г/моль (мг/ммоль)
81 г/моль (мг/ммоль)
Мэк[Mg(НСO3)2] = 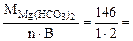 73 г/моль (мг/ммоль)
73 г/моль (мг/ммоль)
Массу гашеной извести, необходимую для устранения временной жесткости рассчитываем по уравнениям реакции:
Ca(HCO3)2 + Ca(OH)2 = 2CaCO3↓ + 2H2O
 162 г 74 г
162 г 74 г 
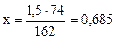 г
г
 1,5 г х
1,5 г х
Mg(HCO3)2 + 2Ca(OH)2 = Mg(OH)2↓ + 2CaCO3↓ + 2H2O
 146 г 2·74 г
146 г 2·74 г 
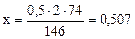 г
г
 0,5 г х
0,5 г х
 0,685 + 0,507 = 1,192 г.
0,685 + 0,507 = 1,192 г.
Ответ:  1,192 г.
1,192 г.





 =16,2 г
=16,2 г
 = 140 л
= 140 л
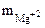 = 2,92 г
Ж -?
= 2,92 г
Ж -?
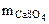 -?
-?
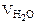 = 10 л
Ж = 5 ммоль/л
= 10 л
Ж = 5 ммоль/л
 = 12 см3
= 12 см3
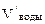 = 40 л
Сэк(HCl) = 0,15 моль/л
= 40 л
Сэк(HCl) = 0,15 моль/л
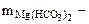 ?
Ж -?
?
Ж -?
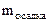 = 28 мг = 0,028 г.
= 28 мг = 0,028 г.
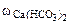 =0,015%
=0,015%
 =0,005%
=0,005%
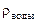 = 1 г/см3
= 1 г/см3
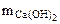 -?
-?

