УРОВЕНЬ A
1. Рассчитать изменение стандартных энтальпии и энтропии химической реакции:
4HCl(г) + O2(г) = 2Н2О(г) + 2Сl2(г) (4.1)
| Дано: Уравнения химической реакции. |


|
РЕШЕНИЕ:
Изменение стандартных энтальпии и энтропии химической реакции рассчитываем на основании первого следствия из закона Гесса:
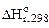 =
= 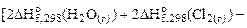
- 
 =
= 
- 
где  и
и  - стандартные энтальпии образования и энтропии веществ, которые находим из таблицы стандартных термодинамических величин
- стандартные энтальпии образования и энтропии веществ, которые находим из таблицы стандартных термодинамических величин
| 4HCl(г) | + О2(г) | = 2Н2О(г) | + 2Сl2(г) | |
 ,
кДж/моль ,
кДж/моль
| 4(-92,3) | 2(-41,84) | ||
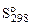 ,
Дж/(мольК) ,
Дж/(мольК)
| 4(186,8) | 2(188,7) | 2(222,9) |
тогда:
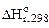 = 2(-241,84) - 4(-92,3) = -114,48 кДж
= 2(-241,84) - 4(-92,3) = -114,48 кДж
 = (2·188,7 + 2·222,9) - (4·186,8 + 205) = -129 Дж/К
= (2·188,7 + 2·222,9) - (4·186,8 + 205) = -129 Дж/К
Ответ: 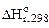 = -114,48 кДж,
= -114,48 кДж,  = -129 Дж/К
= -129 Дж/К
2. Стандартная энтальпия реакции сгорания метанола (СН3ОН) равна (-726,64) кДж/моль. Написать термохимическое уравнение сгорания метанола и вычислить стандартную энтальпию его образования.
Дано:
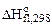 (CH3ОН(ж)) = =-726,64 кДж/моль (CH3ОН(ж)) = =-726,64 кДж/моль
|
 (CH3ОН(ж)) -? (CH3ОН(ж)) -?
|
РЕШЕНИЕ:
Стандартной энтальпией сгорания вещества 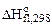 (В) называется тепловой эффект реакции полного сгорания 1 моль органического вещества до СО2(г) и Н2О(ж) при стандартных условиях.
(В) называется тепловой эффект реакции полного сгорания 1 моль органического вещества до СО2(г) и Н2О(ж) при стандартных условиях.
Составляем термохимическое уравнение сгорания 1 моль метанола:
1CH3ОН(ж)+3/2О2(г)=СО2(г)+2Н2О(ж) 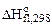 = -726,64 кДж/моль
= -726,64 кДж/моль
Следовательно, для данной реакции 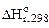 = -726,64 кДж
= -726,64 кДж
Для определения  (CH3ОН(ж)) используем первое следствие из закона Гесса для этой же реакции:
(CH3ОН(ж)) используем первое следствие из закона Гесса для этой же реакции:
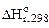 =
=  (СО2(г)) +
(СО2(г)) +  (Н2О(ж)) -
(Н2О(ж)) -
-  (CH3ОН(ж)) -
(CH3ОН(ж)) -  (О2(г)).
(О2(г)).
Откуда:  (CH3ОН(ж)) =
(CH3ОН(ж)) =  (СО2(г))+
(СО2(г))+
+  (Н2О(ж)) -
(Н2О(ж)) - 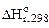 –
–  (О2(г))
(О2(г))
Значения стандартных энтальпий образования  (В) веществ берем из таблицы стандартных термодинамических величин.
(В) веществ берем из таблицы стандартных термодинамических величин.
 (CH3ОН(ж)) = 1(-393,5) +2(-285,8)-(- 726,64) =
(CH3ОН(ж)) = 1(-393,5) +2(-285,8)-(- 726,64) =
= -238,46 кДж/моль.
Ответ:  (CH3ОН(ж)) = -238,46 кДж/моль.
(CH3ОН(ж)) = -238,46 кДж/моль.
3. Можно ли при стандартных условиях восстановить водородом оксид хрома (III), алюминием оксид марганца (II), оксидом углерода (II) оксид ртути (II). Ответ подтвердить расчетом 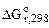 по значениям
по значениям  (В).
(В).
| Дано: Cr2O3 + H2 MnO + Al HgO + CO |
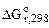 -? -?
|
РЕШЕНИЕ:
Возможность самопроизвольного про-
текания реакции при заданных усло-
виях определяется знаком величины
энергии Гиббса для соответствующей
реакции – если 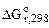 < 0, то самопроизвольное протекание реакции возможно, если же
< 0, то самопроизвольное протекание реакции возможно, если же
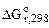 > 0, то протекание данной реакции невозможно.
> 0, то протекание данной реакции невозможно.
Значения 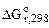 определяем по I следствию из закона Гесса
определяем по I следствию из закона Гесса
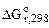 = Σni
= Σni  (В) - Σ nj
(В) - Σ nj 
продуктов исходных
реакции веществ
Значения  (В) находим из таблицы.
(В) находим из таблицы.
Записываем уравнения протекающих реакций
Cr2O3(к) + 3Н2(г) = 2Cr(к) + 3Н2О(ж) (4.1)
 -1059 0 0 3(-237,3)
-1059 0 0 3(-237,3)
кДж/моль
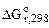 = 3(-237,3) – (-1059) = 374,1 кДж
= 3(-237,3) – (-1059) = 374,1 кДж
Поскольку 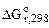 величина положительная, то протекание реакции (4.1) невозможно.
величина положительная, то протекание реакции (4.1) невозможно.
3MnO(к) + 2Al(к) = Al2O3(к) + 3Mn(к) (4.2)
 3(-362,85) 0 -1582 0
3(-362,85) 0 -1582 0
кДж/моль
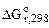 = (-1582) – 3(-362,85) = - 493,45 кДж
= (-1582) – 3(-362,85) = - 493,45 кДж
Поскольку 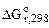 величина отрицательная, то протекание реакции (4.2) возможно.
величина отрицательная, то протекание реакции (4.2) возможно.
HgO(к) + CO(г) = СО2(г) + Hg(ж) (4.3)
 -58,4 - 137,1 -394,4 0
-58,4 - 137,1 -394,4 0
кДж/моль
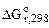 = (-394,4) – [(-58,4) + (-137,1)] = - 194,9 кДж
= (-394,4) – [(-58,4) + (-137,1)] = - 194,9 кДж
Поскольку 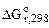 величина отрицательная, то протекание реак-ции (4.3) возможно.
величина отрицательная, то протекание реак-ции (4.3) возможно.
Аналогично определяется возможность протекания реакции в случае использования в качестве восстановителя магния.
УРОВЕНЬ B
1. Вычислить стандартную энтальпию растворения NaOH в воде, если при растворении 10 г NaOH в 250 мл воды температура раствора повысилась от 200С до 29,70С. Удельная теплоемкость раствора равна 3,99 Дж/(г·К).
Дано:

|
 (NaOH) -? (NaOH) -?
|
РЕШЕНИЕ:
Стандартную энтальпию растворения рассчитываем по уравнению:
 (NaOH) =
(NaOH) =
=  , (кДж/моль)
, (кДж/моль)
где МNaOH - молярная масса NaOH, г/моль,
Qp = mр-ра·Ср(р-ра)·( ), -количество выделившейся теплоты при растворении NaOH, Дж
), -количество выделившейся теплоты при растворении NaOH, Дж
mр-ра - масса раствора, г;
Ср(р-ра) – теплоемкость раствора, кДж/(г×К)
1000 - пересчет Дж в кДж.
Для рассматриваемой задачи 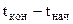 = 29,7-20 = + 9,70. При этом знак "+" указывает на повышение температуры при растворении, а знак "-" - на понижение температуры.
= 29,7-20 = + 9,70. При этом знак "+" указывает на повышение температуры при растворении, а знак "-" - на понижение температуры.
mр-ра = m  + m
+ m  = 10+250 = 260 г.
= 10+250 = 260 г.
Тогда: QР = (10 + 250)·3,99·9,7 = 10062,8 Дж,
 (NaOH) =
(NaOH) =  ,
,
Ответ:  (NaOH) = - 40,24 кДж/моль.
(NaOH) = - 40,24 кДж/моль.
2. Найти массу метана, при полном сгорании, которого выделяется теплота, достаточная для нагревания 30 л воды от 20 до 90оС. Мольная теплоемкость воды равна 75,3 Дж/мольК. КПД теплового агрегата составляет 40%.
Дано:
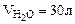

 Ср = 75,3 Дж/мольК
КПД = 40%
Ср = 75,3 Дж/мольК
КПД = 40%
|

|
РЕШЕНИЕ:
Определяем количество теплоты, необходимое для нагревания 30 л воды от 20 до 90оС.

т.к.  =
= 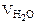

 = 18 г/моль ∆t = 90 – 30 = 60К
= 18 г/моль ∆t = 90 – 30 = 60К

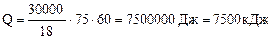
Учитывая КПД теплового агрегата потребуется
 теплоты
теплоты
Такую теплоту необходимо получить при сжигании метана. Тепловой эффект реакции горения метана получим используя I следствие из закона Гесса
 = Σni
= Σni  (В) - Σ nj
(В) - Σ nj 
продуктов исходных
реакции веществ
Уравнение горения метана
СН4(г) + О2(г) = СО2(г) + 2Н2О(г)
 -74,9 0 -393,5 2(-241,84)
-74,9 0 -393,5 2(-241,84)
кДж/моль
 = (-393,5) + 2(-241,84) –(-74,9) = - 802,28 кДж
= (-393,5) + 2(-241,84) –(-74,9) = - 802,28 кДж
Так как при сгорании одного моль метана выделяется 802,28кДж, то определяем количество моль метана, которое необходимо для получения 18750 кДж теплоты.

Определяем массу метана

Ответ: 
3. Испльзуя следующие термохимические уравнения:
2CO(г) + О2(г) = 2CO2(г)  = - 566 кДж (4.10)
= - 566 кДж (4.10)
2Fe(к) + O2(г) = 2FeO(к)  = - 529,6 кДж (4.11)
= - 529,6 кДж (4.11)
4FeO(к) + О2(г) = 2Fe2O3(к)  =- 585,2 кДж (4.12)
=- 585,2 кДж (4.12)






