60 кДж/моль. Во сколько раз изменится скорость этой реакции:
а) при повышении температуры от 320 до 360К?
б) если она протекает при 298 К в присутствии катализатора ( = 48 кДж/моль)?
= 48 кДж/моль)?

| РЕШЕНИЕ:
а) Из уравнения Аррениуса получаем:
 = = 
 =
= =
= 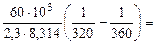 1,09 1,09
|
где 103 – коэффициент пересчета кДж в Дж, тогда
 =101,09 = 12,3 раза;
=101,09 = 12,3 раза;
б) зависимость скорости реакции от наличия катализатора выражается уравнением:
 =
= 
 2,11
2,11
откуда  = 102,11 = 129.
= 102,11 = 129.
Ответ:
а) при повышении температуры от 320 до 360К скорость реакции возрастает в 12,3 раза;
б) в присутствии катализатора скорость реакции возрастает в 129 раз.
УРОВЕНЬ В
1. При погружении цинковой пластины в раствор хлороводородной кислоты за одно и то же время при температуре 293 К выделилось 8 см3 водорода, а при температуре 303 К – 13 см3 водорода. Водород собран над водой при давлении 740 мм рт. ст. Рассчитать энергию активации протекающей реакции. Давление паров воды при температуре 293 К составляет 17,54 мм рт. ст., а при температуре 303 К - 31,82 мм рт. ст.
| РЕШЕНИЕ:
Zn + 2HCl = ZnCl2 + H2↑
Из уравнения
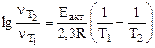 выражаем
энергию активации выражаем
энергию активации
 (5.3) (5.3)
|
Скорость гетерогенной реакции определяем по уравнению:
 ,моль/(см2·мин) (5.4)
,моль/(см2·мин) (5.4)
где  - количество моль выделяющегося водорода;
- количество моль выделяющегося водорода;
S – площадь цинковой пластины, см2;
τ – время протекания реакции, мин.
Определяем отношение скоростей выделения водорода при двух различных температурах (площадь пластины и время протекания реакции постоянны):
 =
=  :
:  =
=  (5.5)
(5.5)
По уравнению Менделеева-Клапейрона
P  V
V  = n
= n  R T, а так как P
R T, а так как P  = (Р – P
= (Р – P  ), то
), то
(P – P  )V
)V  = n
= n  R T, (5.6)
R T, (5.6)
где Р – общее давление газов, мм. рт. ст;
P  - давление насыщенного водяного пара при температуре опыта, мм. рт. ст.;
- давление насыщенного водяного пара при температуре опыта, мм. рт. ст.;
V  - объем выделившегося водорода, см3;
- объем выделившегося водорода, см3;
Т - температура опыта, К;
R -универсальная газовая постоянная.
Из уравнения (5.6) n  = (Р – P
= (Р – P  ) V
) V  / R T (5.7)
/ R T (5.7)
Подставив выражение (5.7) для температур Т1 и Т2 в отношение (5.5), получим
 =
=  :
: 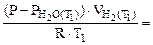
= 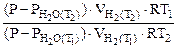

Откуда  = 31834 Дж/моль =
= 31834 Дж/моль =
= 31,83 кДж/моль
Ответ:  = 31,83 кДж/моль.
= 31,83 кДж/моль.
2. На основании принципа Ле Шателье определить, в каком направлении сместится равновесие:
2SO2(г) + O2(г) <=> 2SO3(г), ΔrH0(298K) = -284,2 кДж, при:
а) понижении температуры, б) повышении концентрации SO3, в) повышении давления. Записать выражение для константы равновесия реакции. Ответы обосновать.
Дано:
Уравнение реакции
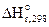 =-284,2 кДж =-284,2 кДж
 Определить направление смещения равновесия при изменении Т, Р, С
Определить направление смещения равновесия при изменении Т, Р, С 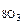
| РЕШЕНИЕ: Выражение константы равновесия реакции может быть приведено в зависимости от равновесных парциальных давлений газообразных участников реакции (5.8) или от их равновесных концентраций (5.9): |
 , (5.8)
, (5.8)  . (5.9)
. (5.9)
а) Выражение зависимости константы равновесия от термодинамических характеристик реакции имеет вид:
-2,3RTlgKP = ΔrH0(298K) – T . ΔrS0(298K), откуда
 . Смещение равновесия реакции при изменении температуры зависит от знака изменения стандартной энтальпии реакции ΔrH0(298K). Так как ΔrH0(298K)<0, то при
. Смещение равновесия реакции при изменении температуры зависит от знака изменения стандартной энтальпии реакции ΔrH0(298K). Так как ΔrH0(298K)<0, то при 
понижении температуры показатель степени будет возрастать, а значит и значение Кр будет возрастать.
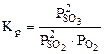 возрастает при увеличении числителя и уменьшении знаменателя, а это возможно при протекании прямой реакции, т.е равновесие 2SO2(г) + O2(г) <=> 2SO3(г) будет смещено вправо (→).
возрастает при увеличении числителя и уменьшении знаменателя, а это возможно при протекании прямой реакции, т.е равновесие 2SO2(г) + O2(г) <=> 2SO3(г) будет смещено вправо (→).
б) Константа равновесия  остается величиной постоянной при любом изменении концентрации участников реакции. Поэтому при повышении концентрации SO3 (числитель) при неизменном значении КС должны автоматически возрасти концентрации исходных веществ (знаменатель), а это возможно при протекании обратной реакции, т.е. в этом случае равновесие сместится влево (←).
остается величиной постоянной при любом изменении концентрации участников реакции. Поэтому при повышении концентрации SO3 (числитель) при неизменном значении КС должны автоматически возрасти концентрации исходных веществ (знаменатель), а это возможно при протекании обратной реакции, т.е. в этом случае равновесие сместится влево (←).
в) В соответствии с принципом Ле Шателье увеличение давления смещает равновесие в сторону той реакции, которая сопровождается уменьшением давления или уменьшением суммарного числа моль газообразных веществ.
 (газ)
(газ)
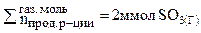 = 2моль(газ)
= 2моль(газ)
Следовательно, при повышении давления равновесие системы смещается вправо (→).
3. Вычислить константу равновесия для гомогенной системы 4NH3(г) +3O2(г) <=> 2N2(г) + 6H2O(г), если исходные концентрации NH3(г) и O2(г) соответственно равны 6,0 и 5,0 моль/л, а равновесная концентрация N2(г) равна 1,8 моль/л.

| РЕШЕНИЕ:
 . (5.11)
Расчет количеств прореагировавших и образовавшихся при установлении равновесия веществ проводится по уравнению реакции.
Равновесная концентрация исходных . (5.11)
Расчет количеств прореагировавших и образовавшихся при установлении равновесия веществ проводится по уравнению реакции.
Равновесная концентрация исходных
|
веществ равна разности исходной концентрации и концентрации прореагировавших веществ. Равновесная концентрация продуктов реакции равна количеству образовавшихся продуктов реакции, т.к. в исходном состоянии их количество было равно нулю.
Обозначим через х количество моль прореагировавших или образовавшихся веществ. Тогда с учетом коэффициентов в уравнении реакции отношения концентраций во второй строке под уравнением реакции равны: ( :
:  :
:  :
: 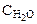 = 4:3:2:6).
= 4:3:2:6).
Последовательность расчетов равновесных концентраций веществ, участвующих в реакции, показана ниже:
| 4NH3 + 3O2 <=> 2N2 + 6H2O | |||||
| Исходная концентрация веществ, моль/л |
 6,0
6,0
| 5,0 | 
|

| |
| Прореагирова-ло, моль/л |
- 4х

|
- 3х 
| Образо-валось, моль/л |
+ 2х

|
 + 6х + 6х

|
| Равновесная концентрация веществ, моль/л | 6,0-4х | 5,0-3х | 2х | 6х |
По условию задачи равновесная концентрация N2 равна 1,8 моль/л. В то же время [N2] = 2х. Следовательно, 2х = 1,8,
а х = 0,9. Тогда равновесные концентрации веществ равны, моль/л:
[NH3] = 6,0-4·0,9 = 2,4 моль/л,
[O2] = 5,0-3·0,9 = 2,3 моль/л,
[N2] = 1,8 моль/л,
[H2O] = 6,0·0,9 = 5,4 моль/л.
 =198,95.
=198,95.
Ответ: Kс = 198,95.
6. ФИЗИКО-ХИМИЧЕСКИЕ СВОЙСТВА
РАСТВОРОВ
УРОВЕНЬ А
1. Вычислить а) температуру кипения, б) температуру замерзания водного раствора, содержащего 0,1 моль сахарозы ( ) в 500 г раствора.
) в 500 г раствора.
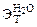 =0,52 кг·К/моль,
=0,52 кг·К/моль,  = 1,86 кг·К/моль
= 1,86 кг·К/моль

| РЕШЕНИЕ:
а) Температура кипения водного раствора сахарозы рассчитывается по уравнению:
tкип р-ра =  + +  =
= 1000С + =
= 1000С +  ,
∆tкип = ,
∆tкип = 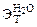 ·cm ( ·cm ( ),
cm( ),
cm( ) – моляльность сахарозы в растворе, моль/кг. ) – моляльность сахарозы в растворе, моль/кг.
|
cm( ) =
) = 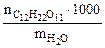 , (6.1)
, (6.1)
где  - количество растворенной сахарозы, моль.
- количество растворенной сахарозы, моль.
 =
=  , г
, г
cm( ) =
) =  =
=
 моль/кг,
моль/кг,
М  = 342 г/моль
= 342 г/моль
 = 0,52∙0,21 = 0,110С
= 0,52∙0,21 = 0,110С

 = 1000С + 0,110С = 100,110С
= 1000С + 0,110С = 100,110С
б) Температура замерзания водного раствора рассчитывается по уравнению:

 =
=  -
-  = 00С -
= 00С -  ,
,
 =
=  ·cm (
·cm ( ),
),
 = 1,86∙0,21 = 0,390С
= 1,86∙0,21 = 0,390С

 = 00С - 0,390С = -0,390С
= 00С - 0,390С = -0,390С
Ответ: 
 = 100,110С
= 100,110С 
 = - 0,390С
= - 0,390С
2. В 100 г воды содержится 2,3 г неэлектролита. Раствор обладает при 250С осмотическим давлением, равным 618,5 кПа. Определить молярную массу неэлектролита. Плотность раствора принять равной 1,05 г/см3.

| РЕШЕНИЕ
π =  ∙R∙T, кПа (6.2)
где mВ – масса растворенного неэлектролита, г
МВ – молярная масса растворенного неэлектролита, г/моль.
R = 8,314 л∙кПа/(моль∙К)
Т = 273 + to
Vр-ра = mр-ра/ρр-ра = (mB + m ∙R∙T, кПа (6.2)
где mВ – масса растворенного неэлектролита, г
МВ – молярная масса растворенного неэлектролита, г/моль.
R = 8,314 л∙кПа/(моль∙К)
Т = 273 + to
Vр-ра = mр-ра/ρр-ра = (mB + m 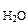 )/ρр-ра =
= )/ρр-ра =
= 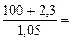 97,43 см3 = 0,097 л 97,43 см3 = 0,097 л
|
МВ =  = 94,98 г/моль
= 94,98 г/моль
Ответ: молярная масса растворенного вещества равна
94,98 г/моль.
3. Определить массу воды, которую необходимо прибавить к 10 г карбамида CO(NH2)2, чтобы давление пара над полученным раствором при температуре 298К составило 3,066 кПа. Давление пара над водой при этой температуре равно 3,166 кПа.

| Решение
Давление насыщенного пара воды над раствором электролита равно:
 (6.3) (6.3)
|
Откуда
где  - количество воды, моль;
- количество воды, моль;
 - количество карбамида, моль.
- количество карбамида, моль.
Откуда 
 =
= 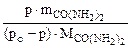 =
=
=  моль
моль
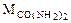 = 12 + 16 + 2(14 + 2) = 60 г/моль.
= 12 + 16 + 2(14 + 2) = 60 г/моль.
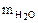 =
=  · М
· М  = 5,11 · 18 = 91,98 г
= 5,11 · 18 = 91,98 г
Ответ: 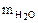 = 91,98 г.
= 91,98 г.
УРОВЕНЬ В
1. Определить давление насыщенного пара воды над 0,05 М раствором хлорида кальция (ρ = 1,041 г/см3) при 301К, если давление насыщенного пара над водой при этой температуре равно 3,78 кПа. Кажущаяся степень диссоциации хлорида кальция равна 0,85.

| РЕШЕНИЕ:
Давление насыщенного пара воды над раствором электролита равно:

 , (6.4) , (6.4)
|
где  - количество воды, моль,
- количество воды, моль,
 - количество CaCl2, моль
- количество CaCl2, моль
i – изотонический коэффициент.
α =  , i = α (к-1) + 1 (6.5)
, i = α (к-1) + 1 (6.5)
где α – кажущаяся степень диссоциации CaCl2
к – общее количество ионов, образующихся при полной
диссоциации одной молекулы CaCl2.
CaCl2 = Сa2+ + 2Cl-
к = 3
i = 0,85(3-1)+1 = 2,7

 , моль/л, (6.6)
, моль/л, (6.6)
oткуда  , г
, г
 = 111 г/моль
= 111 г/моль
Для расчета берем 1л раствора

mр-ра = Vр-ра·ρ = 1000∙1,041 = 1041г.
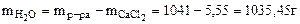
р = 3,78∙  = 3,77 кПа.
= 3,77 кПа.
Ответ: давление насыщенного пара над раствором равно 3,77 кПа.
2. Определить осмотическое давление 1,5% раствора карбоната натрия при 250С. Плотность раствора равна 1,015 г/см3. Кажущаяся степень диссоциации карбоната натрия равна 0,9.

| РЕШЕНИЕ:
π = i∙  ∙R∙T, кПа (6.7)
α = ∙R∙T, кПа (6.7)
α =  ; i = α(к-1)+1 ; i = α(к-1)+1
|
где α - кажущаяся степень диссоциации,
к – общее количество ионов, образующихся при полной
диссоциации одной молекулы Na2CO3
Na2CO3 = 2Na+ + CO32-
к = 3, тогда i = 0,9(3-1)+1 = 2,8
 - молярная концентрация Na2CO3, моль/л
- молярная концентрация Na2CO3, моль/л
 =
=  , моль/л
, моль/л
В 100 г 1,5%-ного раствора содержится 1,5 г Na2CO3
Vр-ра =  =
=  = 98,5см3 = 0,0985 л.
= 98,5см3 = 0,0985 л.
 =
=  = 0,144 моль/л
= 0,144 моль/л
π = 2,8∙0,144∙8,314∙298 = 998,96 кПа
Ответ: осмотическое давление 1,5% раствора Na2CO3 равно 998,96 кПа.
3. Определить кажущуюся степень диссоциации соли, если водный раствор хлорида алюминия с массовой долей 1,5% кристаллизуется (замерзает) при температуре (-0,690С).
 =1,86 кг·К/моль.
=1,86 кг·К/моль.

| РЕШЕНИЕ:
α =  ,
где к – общее количество ионов, образующихся при полной диссоциации одной молекулы AlCl3 ,
где к – общее количество ионов, образующихся при полной диссоциации одной молекулы AlCl3
|
AlCl3 = Al3+ + 3Cl- к = 4
∆tзам = i∙  ∙сm(AlCl3), (6.8)
∙сm(AlCl3), (6.8)

где сm(AlCl3) =  , моль/кг
, моль/кг
В 100г 1,5%-ного раствора содержится 1,5г AlCl3 и 98,5 г воды.
сm(AlCl3) =  = 0,114 моль/кг,
= 0,114 моль/кг,
 = 133,5 г/моль
= 133,5 г/моль
 = 3,25
= 3,25
α = 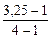 = 0,75 (75%)
= 0,75 (75%)
Ответ: кажущаяся степень диссоциации AlCl3 равна 0,75(75%).
 7. РАСТВОРЫ СИЛЬНЫХ И СЛАБЫХ ЭЛЕКТРОЛИТОВ
7. РАСТВОРЫ СИЛЬНЫХ И СЛАБЫХ ЭЛЕКТРОЛИТОВ
УРОВЕНЬ А
1. Вычислить рН следующих водных растворов:
А) 0,02 М HCl; б) 0,2 М KOH
| Дано: cHCl = 0,02 М cKOH = 0,2 М |
| pH -? |
РЕШЕНИЕ:
а) HCl = H+ + Cl- (7.1)
рН = - lg c 
c  = cНСl·α·n
= cНСl·α·n  ,
,
HCl - сильный электролит, α = 1;
n  - число Н+, образовавшихся при диссоциации одной молекулы НСl;
- число Н+, образовавшихся при диссоциации одной молекулы НСl;
n  = 1, тогда c
= 1, тогда c  = c
= c  = 0,02 моль/л = 2·10-2 моль/л.
= 0,02 моль/л = 2·10-2 моль/л.
рН = - lg 2·10-2 = 1,7.
б) KOH = К+ + ОН-, (7.2)
рН = 14 - рОН; рОН = - lg c  ;
;
c  = c
= c  ·α·n
·α·n  ,
,
КОН - сильный электролит, α = 1;
n  - число ОН-, образовавшихся при диссоциации одной молекулы КОН, n
- число ОН-, образовавшихся при диссоциации одной молекулы КОН, n  = 1, тогда
= 1, тогда
c  = cKOH = 2·10-1 моль/л, рОН = - lg (2·10-1) = 0,7
= cKOH = 2·10-1 моль/л, рОН = - lg (2·10-1) = 0,7
рН = 14 - 0,7 = 13,3.
Ответ: рН 0,02 М HCl равно 1,7;
рН 0,2 М КОН равно 13,3.





 -?
б)
-?
б) 
 = 8 см3,
= 8 см3,

 = 13 см3,
P
= 13 см3,
P  = 21,07 мм рт.ст.
P
= 21,07 мм рт.ст.
P 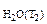 =31,82 мм рт.ст.
P = 740 мм рт.ст.
=31,82 мм рт.ст.
P = 740 мм рт.ст.
 τ
τ  -?
-?

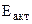 -?
-? моль/л,
моль/л,
 моль/л,
[N2] = 1,8 моль/л
Kс -?
моль/л,
[N2] = 1,8 моль/л
Kс -?
 mр-ра = 500 г
mр-ра = 500 г
 = 100 г
π = 618,5 кПа
ρр-ра = 1,05г/см3
МВ -?
= 100 г
π = 618,5 кПа
ρр-ра = 1,05г/см3
МВ -?
 моль/л
T = 301К
ρ = 1,041 г/см3
α = 0,85
рo = 3,78 кПа
р -?
моль/л
T = 301К
ρ = 1,041 г/см3
α = 0,85
рo = 3,78 кПа
р -?
 = 1,5%
t = 250С
ρ = 1,015 г/см3
α = 0,9
π -?
= 1,5%
t = 250С
ρ = 1,015 г/см3
α = 0,9
π -?
 = 1,5%
tзам р-ра = -0,690С
= 1,5%
tзам р-ра = -0,690С

