БЕЛОРУССКИЙ НАЦИОНАЛЬНЫЙ ТЕХНИЧЕСКИЙ УНИВЕРСИТЕТ
═══════════════════════════════════════
Кафедра химии
ЗАДАЧИ ЛАБОРАТОРНОГО
КОНТРОЛЯ ПО ХИМИИ
Учебно-методическое пособие по дисциплине «Химия»
Для студентов I курса
Под редакцией А.Р. Цыганова
Электронный учебный материал
М и н с к 2015
УДК 546.66.015
Авторы:
Г.А. Бурак, Г.К. Глушонок, В.А. Горбунова, Н.В. Зык, Н.Г. Кирюшина, Н.А. Кречко, Д.И. Медведев, А.А. Меженцев, И.Б. Проворова, Л.М. Слепнева, Ю.В. Шагойко, В.Н. Яглов
Задачи лабораторного контроля по химии / Учебно-методическое пособие по дисциплине «Химия» для студентов I курса. - Мн.: БНТУ, 2015
В данном пособии представлены решения типовых задач различного уровня сложности по основным разделам курса химии, который излагается студентам на лекциях. В задачах даны термины и условные обозначения, применяемые в Международной системе единиц.
Рецензенты: О.Ф. Краецкая, к.х.н., доцент кафедры
«Промышленная теплоэнергетика и тепло техника» БНТУ;
А.Г. Слуцкий, к.т.н., доцент кафедры
«Металлургия литейных сплавов» БНТУ
УДК 546.66.015
© Бурак Г.А. и др.
ВВЕДЕНИЕ
При изучении курса химии предусмотрены следующие виды занятий: лекции, практические и лабораторные занятия. Лекции читаются в соответствии с рабочей программой, вопросы которой представлены в приложении 2.
На первом занятии студент получает номер варианта своего индивидуального домашнего задания, которое он обязан выполнить к каждой лабораторной работе (см. приложение 1).
Домашнее задание включает краткий конспект по вопросам предстоящей лабораторной работы и задачи, соответствующие полученному студентом варианту.
Вопросы для конспектирования представлены в плане лабораторных работ, вывешенном в лаборатории.
Каждая лабораторная работа начинается с проверки домашнего задания. Затем проводится лабораторная работа.
Лабораторная работа считается зачтенной если:
а) студент выполнил домашнее задание (не менее 1 балла);
б) выполнил лабораторную работу с ошибкой менее 10%, оформил и сдал в установленное время отчет по лабораторной работе (2 балла);
в) защитил лабораторную работу. Защита лабораторной работы проводится путем решения задач ур. А (не менее 1 балла) и ур. В (до 4 баллов). Задачу ур. В студент может получить только, если задача ур. А решена не более, чем за 10 минут.
Преподаватель имеет право снижать оценку в случае некачественного выполнения и оформления отдельных видов работ. Суммарная минимальная оценка для получения зачета по лабораторной работе составляет 4 балла при условии хотя бы частичного выполнения всех трех слагаемых лабораторной работы: домашнего задания, лабораторной работы и ее защиты. При отсутствии оценки хотя бы по одному из этих трех слагаемых лабораторная работа не засчитывается.
Если студент не представил выполненное домашнее задание или не решил зачетную задачу или отчет по лабораторной работе, то защита этой лабораторной работы переносится на следующее занятие.
Если студент на следующем лабораторном занятии не представил выполненное домашнее задание по предыдущей работе или не представил отчет о предыдущей лабораторной работе, или не защитил предыдущую лабораторную работу, то эта лабораторная работа ему не засчитывается и студент обязан отработать ее полностью заново во вне учебное время в течение двух недель у своего преподавателя после оплаты в кассу университета. Оценка ставится только за зачтенную лабораторную работу. Если лабораторная работа не зачтена, то оценка - 0 баллов.
После выполнения лабораторной работы студенту выставляется оценка.
 1. ОСНОВНЫЕ КЛАССЫ НЕОРГАНИЧЕСКИХ СОЕДИНЕНИЙ
1. ОСНОВНЫЕ КЛАССЫ НЕОРГАНИЧЕСКИХ СОЕДИНЕНИЙ

|
 |  | ||
NO, CO, SiO, N2O
|
|
|
Основные:Кислотные:
Li2O - оксид лития; В2О3 - оксид бора;
MgO - оксид магния; СО2 - оксид углерода(IV);
МnО - оксид марганца(II). Mn2О7 - оксид марганца(VII)
FeO –оксид железа (II) CrO3 –оксид хрома (VI)
Амфотерные
BeO - оксид бериллия; ZnO - оксид цинка;
Al2O3 - оксид алюминия; SnO - оксид олова (II);
Cr2O3 - оксид хрома (III); PbO - оксид свинца (II).
СВОЙСТВА ОКСИДОВ:
Основные:
CaO + СО2 = СаСO3
СuО + H2SO4 = CuSO4 + Н2О;
Na2O +H2O=2NaOH;
СuО + H2 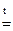 Cu + Н2О Cu + Н2О
| Кислотные: СО2 + СuО = СuСO3 СО2 + NaOН = NaHCO3 СО2 + Ва(ОН)2 = = ВаСО3↓ + Н2О SO3 + Н2О = H2SO4; |
Амфотерные:
ZnO + 2HCl = ZnCl2 + H2O;
ZnO + 2 NaOH 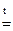 Na2ZnO2 + H2O;
Na2ZnO2 + H2O;
цинкат натрия
ZnO + 2 NaOH + H2O = Na2[Zn(OH)4];
тетрагидроксоцинкат натрия
Al2О3 + 6HCI = 2A1CI3 +3H2O;
А12О3 + Na2CO3 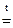 2NaAlO2 + CO2;
2NaAlO2 + CO2;
А12О3 + 2NaOH 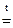 2NaAlO2 + H2O.
2NaAlO2 + H2O.
метаалюминат натрия
А12О3 + 2NaOH + 3 H2O = 2Na[Al(OH)4]
тетрагидроксоалюминат натрия

| LiOH - гидроксид лития; NaOH - гидроксид натрия; КОН - гидроксид калия; RbOH - гидроксид рубидия; CsOH - гидроксид цезия; Са(ОН)2 -гидроксид кальция; Sr(OH)2 - гидроксид стронция; Ва(ОН)2 - гидроксид бария; | Fe(OH)2- гидроксид железа (II); Mg(OH)2 - гидроксид магния Амфотерные гидроксиды: Zn(OH)2 = H2ZnO2 гидроксид цинковая цинка кислота А1(ОН)3 = Н3АlO3 = НАlO2 + Н2О гидроксид ортоалю- метаалю- алюминия миниевая миниевая кислота кислота |
СВОЙСТВА ОСНОВАНИЙ
Cu(OH)2 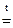 CuO + H2O.
CuO + H2O.
КОН +HCl = KCl + H2O;
Са(ОН)2 + CO2 = CaCO3↓ + H2O;
2NaOH + CuSO4 = Cu(OH)2↓ + Na2SO4;
Al(OH)3 + 3HCl = AlCl3 + 3H2O;
Al(OH)3 + NaOH 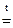 NaAlO2 + 2H2O;
NaAlO2 + 2H2O;
метаалюминат натрия
Al(OH)3 + 3NaOH 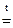 Na3AlO3 + 3H2O;
Na3AlO3 + 3H2O;
ортоалюминат натрия
Al(OH)3 + NaOH = Na[Al(OH)4];
тетрагидроксоалюминат натрия
Al(OH)3 + 3NaOH = Na3[Al(OH)6];
гексагидроксоалюминат натрия
|
 | |||
 | |||
| HF - фтороводородная HCl - хлороводородная; HJ - иодоводородная; НВг - бромоводородная; H2S - сероводородная; HCN - циановодородная. HSCN - родановодородная | H3BО3 - ортоборная; HBО2 - метаборная Н2СО3 - угольная; H4SiO4 - ортокремниевая, H2SiO3 - метакремниевая НNO3 - азотная; HNO2 - азотистая; Н3РО4 - ортофосфорная; НРО3 - метафосфорная; H2SO4 - серная; Н2SОз - сернистая; НМnО4 - марганцовая; НClO4 - хлорная; CH3COOH - уксусная; HCOOH –муравьиная |
СВОЙСТВА КИСЛОТ:
2НС1 + СаСO3 = СaCI2 + H2O + CO2↑;
2HC1 + Zn = ZnCI2 + H2↑;
H2SO4 + CuO = CuSO4 + H2O;
H2SO4 + Ba(OH)2 = BaSO4↓ + 2H2O;
H2SO4 + K2SiO3 = H2SiO3↓ + K2SO4;
HC1 + AgNO3 = AgCl↓ + HNO3.
H2 SiO3 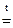 H2O + SiO2
H2O + SiO2
H2SO3 = H2O + SO2
 | |||
 | |||
| 2NaOH + H2SO4 = Na2SO4 + 2H2O; K2SO3 –сульфит калия; Na2SO4 - сульфат натрия; Ni(NO3)2 - нитрат никеля (II); Fe(NO2)3 – нитрит железо (III); K2S - сульфид калия; NH4Cl – хлорид аммония. Сa3(PO4)2 – ортофосфат кальция; КPO3 – метафосфат калия; Na2SiO3 – метасиликат натрия | Fe(OH)3 + 2HNO3 = = FeOH(NO3)2 + 2H2O (CuOH)2SO4 - сульфат гидроксомеди(II); Fe(OH)2NO3 – нитрат дигидроксожелеза (III); ZnOHСl – хлорид гидроксоцинка. |
|
 |
2H3РO4 + Ba(OH)2 = Ba(H2PO4)2 + 2H2O
KHSO3 - гидросульфит калия;
А1(Н2РО4)3 - дигидроортофосфат алюминия
Ca(HCO3)2 – гидрокарбонат кальция;
NaHS – гидросульфид натрия;
SrHPO4 – гидроортофосфат стронция.
Таблица 1.1. Кислоты и их соли.
| Формула кислоты | Формула аниона | Название аниона |
| HBO2 | BO2- | Метаборат |
| H3BO3 | BO33- | Ортоборат |
| Н2SO4 | SO42- | Сульфат |
| H2 SO3 | SO32- | Сульфит |
| HNO3 | NO3- | Нитрат |
| HNO2 | NO2- | Нитрит |
| H2CO3 | CO32- | Карбонат |
| H2SiO3 | SiO32- | Метасиликат |
| H3PO4 | PO43- | Ортофосфат |
| HPO3 | PO3- | Метафосфат |
| HMnO4 | MnO4- | Перманганат |
| HF | F- | Фторид |
| HСl | Cl- | Хлорид |
| HBr | Br- | Бромид |
| HI | I- | Иодид |
| H2S | S2- | Сульфид |
| HCN | CN- | Цианид |
| CH3COOH | CH3COO- | Ацетат |
| HCOOH | HCOO- | Формиат |
СВОЙСТВА СОЛЕЙ
Hg(NO3)2 + Zn = Zn(NO3)2 + Hg;
CuSO4 + 2 NaOH = Cu(OH)2↓ + Na2SO4;
Fe(NO3)2 + H2S = FeS↓ + 2HNO3;
CaCl2 + Na2CO3 = CaCO3↓ + 2NaCl;
KHSO4+ KOH = K2S04 + H2O;
CuOHCl + HCl = CuCI2 + H2O;
CuSO4·5H2O 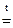 CuSO4 + 5H2O.
CuSO4 + 5H2O.

НЕКОТОРЫЕ ПРАВИЛА ОПРЕДЕЛЕНИЯ СТЕПЕНЕЙ ОКИСЛЕНИЯ ЭЛЕМЕНТОВ В ХИМИЧЕСКИХ СОЕДИНЕНИЯХ.
1. Степень окисления атомов в простых веществах равна нулю.
Например; Н2°; N2°, О2°, Na°, Cu°, Fe°, Hg°, Sо, P°, B° и др.
2. Атомы кислорода в основных классах неорганических соединений проявляют степень окисления (-2).
Например: Са+2О-2,S+4O2-2 и т.д.
Исключения: фторид кислорода О+2 F2-1 - степень окисления кислорода (+2), пероксиды - степень окисления кислорода (-1),
Например: Н2+1 О2-1, Ca+2 O2-1.
3. Атом водорода в основных классах неорганических соединений проявляет степень окисления (+ 1).
Например: H2+1S-2, N-3H3+1, Н2+1О-2, K+1O-2H+1, Na+1H+1C+4O  .
.
Исключения: гидриды металлов типа Ca+2H2-1; Na+1H-1; в которых степень окисления водорода (-1).
4. Металлы IA, IIА и IIIA главных подгрупп Периодической системы проявляют степень окисления равную номеру группы в которой находится этот элемент т.е. соответcтвенно (+1), (+2) и (+3) (см. табл.1.2).
Например: Na2+1O-2; Sr+2O-2; Al2+3O3-2.
5. Алгебраическая сумма степеней окисления отдельных атомов, образующих молекулу, с учетом их стехиометрических индексов равна нулю. Например, можно определить степень окисления азота в молекуле НNO3, зная степени окисления кислорода (-2) и водорода (+1): H+1NхО3-2
(+1) + х +(-2)·3 = 0; х = +5
или степень окисления хрома в молекуле К2+1Cr2хО7-2:
(+1) · 2 + х · 2 + (-2)·7 = 0 х = +6
6. Атомы одного и того же элемента в различных соединениях могут иметь разные степени окисления, например:
K+1Mn+7O4-2; H2+1Mn+6O4-2; Mn+4O2-2.
Таблица 1.2. Элементы, имеющие постоянную степень окисления в большинстве соединений
| I | II | III | IV | V | VI |
| H+ | |||||
| Li+1 | Be+2 | B+3 | O-2 | ||
| Na+1 | Mg+2 | Al+3 | |||
| K+1 | Ca+2 Zn+2 | Sc+3 | |||
| Rb+1 Ag+1 | Sr+2 Cd+2 | ||||
| Cs+1 | Ba+2 | ||||
| Fr+1 | Ra+2 |
УРОВЕНЬ А
1. а) Назвать следующие химические соединения и определить степень окисления всех элементов соединений: СО, Mn(OH)2, H2SO4, KHS, Na2CO3, FeOH(NO3)2.
б) Написать формулы следующих химических соединений: оксид свинца (IV), сульфат лития, хлорид гидроксоцинка, дигидроортофосфат алюминия
Ответ:
а) С+2О-2 - оксид углерода (II),
Mn+2 (O-2H+1)2 - гидроксид марганца (II),
Н+12S+4O-23 - сернистая кислота,
К+1Н+1S-2 - гидросульфид калия,
Na2+1С+4О-23 - карбонат натрия,
Fe+3O-2H+1(N+5O-23)2 - нитрат гидроксожелеза (III).
б) Оксид свинца (IV) - PbO2,
сульфат лития - Li2SO4,
хлорид гидроксоцинка – ZnOHCl
дигидроортофосфат алюминия – Al(H2PO4)3
УРОВЕНЬ В
1. Определить количество моль воды в формуле кристаллогидрата Na2CO3·хH2O, если в результате прокаливания его масса изменилась от 1,43 до 0,53 г. Определить объем выделившейся парообразной воды при 200оС и давлении 98,2 кПа.
Дано:

| РЕШЕНИЕ:
При прокаливании кристаллогидрата протекает реакция:
Na2CO3·nH2O 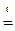 Na2CO3 +nH2O↑ (1.1)
Находим массу воды ( Na2CO3 +nH2O↑ (1.1)
Находим массу воды ( ), выделившейся при разложении кристаллогидрата: ), выделившейся при разложении кристаллогидрата:
|
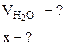
|
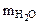 = 1,43 - 0,53 = 0,9 г
= 1,43 - 0,53 = 0,9 г
Расчет n ведем по уравнению реакции (1.1)
 или
или  , тогда
, тогда
х = 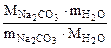 =
= 
где 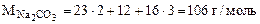
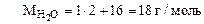
Формула кристаллогидрата Na2CO3·10H2O
Рассчитываем объем выделившейся газообразной воды при
Т = 273 + 200 = 473 К и Р = 98,2 кПа по уравнению Менделеева-Клапейрона

где Р – Па, V – м3, m – кг, М  – кг/моль, R – 8,314 Дж/моль·К,
– кг/моль, R – 8,314 Дж/моль·К,
Т – К.
Откуда 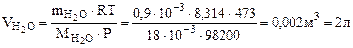
Ответ: формула кристаллогидрата: Na2CO3·10Н2О; 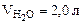
2. Определить состав смеси (% масс.), содержащей карбонат магния (15 г) и оксид магния. Если после прокаливания смеси, полученный продукт вступил в реакцию с ортофосфорной кислотой и образовалось 80 г ортофосфата магния. Составить уравнения реакций.
Дано:
MgO и MgCO3
m 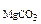 = 15 г
m = 15 г
m 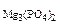 = 80 г = 80 г
| РЕШЕНИЕ:
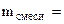  + + 
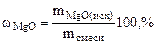 мас. мас.
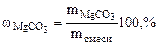 мас. мас.
|
ω 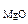 -? ω -? ω 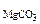 -? -?
|
При прокаливании смеси протекает реакция
MgCO3 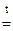 MgO + CO2
MgO + CO2
Тогда по этому уравнению
n 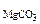 =
=  (из MgCO3) или
(из MgCO3) или 
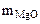 (из MgCO3) =
(из MgCO3) = 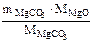 =
= 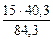 = 7,17 г
= 7,17 г
где 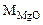 = 24,3 + 16 = 40,3 г/моль
= 24,3 + 16 = 40,3 г/моль
М 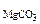 = 24,3 + 12 + 16·3 = 84,3 г/моль
= 24,3 + 12 + 16·3 = 84,3 г/моль
После прокаливания смесь состоит только из MgО
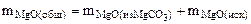
При взаимодействии продукта, полученного после прокаливания с ортофосфорной кислотой, протекает реакция
3MgO(общ.) + 2H3PO4 = Mg3(PO4)2 + 3H2O
Тогда реагирует по уравнению
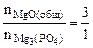 →
→ 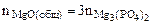
или
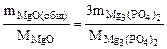
откуда 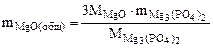
mMgO(общ) = 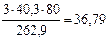 г
г
где М  = 40,3 г/моль, М
= 40,3 г/моль, М  = 262,9 г/моль
= 262,9 г/моль
тогда
mMgO(исх) = mMgO(общ) – mMgO (из MgCO 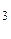 ) = 36,79 г – 7,17 г = 29,62 г
) = 36,79 г – 7,17 г = 29,62 г
Таким образом
mсмеси = mMgO(исх) +  = 29,62 г + 15 г = 44,62 г
= 29,62 г + 15 г = 44,62 г
Состав исходной смеси
ω 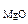 =
= 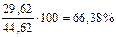 мас.
мас.
ω 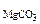 = 100 – 66,38 = 33,62% масс.
= 100 – 66,38 = 33,62% масс.
3. При взаимодействии 1,8 г технического карбоната кальция (мела) с HCl выделилось 400 см3 CO2, собранного над водным раствором NaHCO3 и измеренного при температуре 288 К и давлении 730 мм рт.ст. Давление паров воды при 288 К равно 12,79 мм рт.ст. Определить содержание СаСО3 в техническом карбонате кальция в процентах.
Дано:
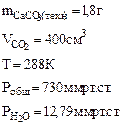
 
| РЕШЕНИЕ:
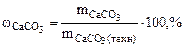 Так как, примеси, содержащиеся в техническом СаСО3 не взаимодействуют с HCl с образованием CO2, то массу чистого карбоната кальция (
Так как, примеси, содержащиеся в техническом СаСО3 не взаимодействуют с HCl с образованием CO2, то массу чистого карбоната кальция ( )
определяем по уравнению реакции (1.4): )
определяем по уравнению реакции (1.4):
|
СаСО3 +2HCl=CaCl2 + H2O+CO2↑ (1.4)

Или  VM(CO2) = 22400 cм3/моль
VM(CO2) = 22400 cм3/моль
тогда 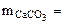
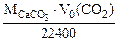 (1.5)
(1.5)
где 
где 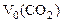 - объем выделившегося СО2, приведенный к нормальным условиям.
- объем выделившегося СО2, приведенный к нормальным условиям.
 Для определения
Для определения  воспользуемся объединенным газовым законом:
воспользуемся объединенным газовым законом:
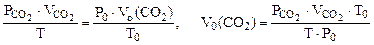
где 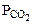 - парциальное давление СО2, так как СО2 собран над водным раствором NaHCO3, то
- парциальное давление СО2, так как СО2 собран над водным раствором NaHCO3, то
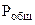 =
= 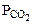 +
+ 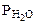 , тогда
, тогда
 мм рт.ст.
мм рт.ст. 
Тo = 273 К,
Рo = 760 мм рт. ст.,
тогда  =
= 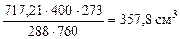
Определяем массу чистого СаСО3 по формуле (1.5):
 =
= 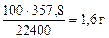 , тогда
, тогда

Ответ: содержание СаСО3 в меле 88,89%.
2. ЭКВИВАЛЕНТ. ЗАКОН ЭКВИВАЛЕНТОВ
УРОВЕНЬ А
1. Написать уравнения реакций взаимодействия хлорида железа (III) с гидроксидом натрия с образованием:
а) хлорида гидроксожелеза;
б) гидроксида железа (III).
В каждой из реакций вычислить эквивалентную массу хлорида железа (III).

| РЕШЕНИЕ:
Мэк (FeCl3) =  , г/моль , г/моль
 - число ионов хлора,
замещенных в данной реакции на гидроксильную группу ОН-.
По реакции а): - число ионов хлора,
замещенных в данной реакции на гидроксильную группу ОН-.
По реакции а):
|
а) FeCl3 + NaOH = FeOHCl2 + NaCl
определяем 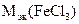 :
:
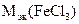 =
= 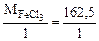 = 162,5 г/моль
= 162,5 г/моль
б) FeCl3 + 3NaOH = Fe(OH)3 + 3NaCl
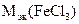 =
=  = 54,17 г/моль
= 54,17 г/моль
Ответ:а) 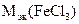 = 106,8 г/моль
= 106,8 г/моль
б) 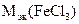 = 54,17 г/моль
= 54,17 г/моль
2. На восстановление 7,2 г оксида потребовалось 2,24 л водорода, измеренного при н.у. Рассчитать эквивалентные массы оксида и металла. Назвать этот металл.

| РЕШЕНИЕ:
По закону эквивалентов
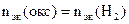
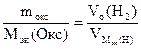
 = 11,2 л/моль – эквивалентный объем водорода = 11,2 л/моль – эквивалентный объем водорода
|
МЭК(Окс) = 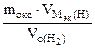 =
= 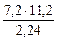 = 36 г/моль
= 36 г/моль
МЭК(Окс) = МЭК(Ме) + МЭК(О)
МЭК(Ме) = МЭК(Окс) – МЭК(О) = 36 - 8 = 28 г/моль
МЭ = МЭК(Ме) · В
Если В = 1 МЭ = 28 г/моль. Такого металла в таблице Д.И. Менделеева нет. Если В = 2 МЭ = 56 г/моль. Следовательно, Ме - Fe
Ответ: Мэк(Окс) = 36 г/моль, Мэк(Ме) = 28 г/моль, Ме – Fe
3. Хлорид некоторого металла массой 0,493 г обработали избытком раствора AgNO3. При этом образовалось 0,86 г AgCl. Вычислить эквивалентную массу эквивалента металла и назвать этот металл.

| РЕШЕНИЕ:
По закону эквивалентов:
 т.е. т.е.
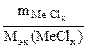 = = 
 = = 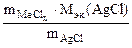
|
где х – валентность Ме, т.к. заряд хлорид иона равен – 1.
Мэк (AgCl) = 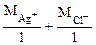 = 108 + 35,5 = 143,5 г/моль
= 108 + 35,5 = 143,5 г/моль
 =
= 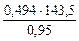 = 74,62 г/моль
= 74,62 г/моль
 =
=  +
+ 

 г/моль
г/моль
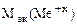 =
= 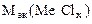 -
- 
 = 74,62 –35,5 = 39,12 г/моль.
= 74,62 –35,5 = 39,12 г/моль.
МЭ =  · В. Если х = В = 1, то МЭ = 39,12 г/моль, что соответствует молярной массе атома калия.
· В. Если х = В = 1, то МЭ = 39,12 г/моль, что соответствует молярной массе атома калия.
Ответ:  = 39,12 г/моль, Ме - К
= 39,12 г/моль, Ме - К
УРОВЕНЬ В
1. При растворении 16,25 г двухвалентного металла в кислоте выделилось 6,52 л водорода, собранного над водой и измеренного при температуре 298К и давлении 730 мм рт. ст. Определить эквивалентную массу металла и назвать металл. Давление паров воды при температуре 298К равно 23,76 мм рт. ст.

| РЕШЕНИЕ:
По закону эквивалентов
 = = 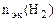
 Мэк(Ме) =
Мэк(Ме) = 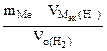 =
= =
= 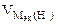 = 11,2 л/моль = 11,2 л/моль
|
По объединенному уравнению газового состояния
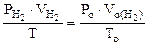
где: То = 273 К, Ро = 760 мм рт.ст.
Робщ = 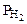 +
+ 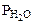 ;
; 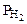 = Робщ -
= Робщ - 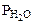
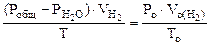
Определяем 
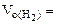
 = 5,55л
= 5,55л
Мэк(Ме) =  =32,79 г/моль
=32,79 г/моль
Мэк(Ме) = 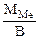 , г/моль, где В – валентность металла
, г/моль, где В – валентность металла
ММе = Мэк(Ме) ∙ В = 32,79 ∙ 2 = 65,58 г/моль, что соответствует молекулярной массе атома цинка.
Ответ: Мэк(Zn) = 32,79 г/моль, металл – Zn
2. При окислении металла израсходовано 3,79 л кислорода, измеренного при 293 К и давлении 740 мм рт.ст. Образовалось 39,43 г оксида. Определить эквивалентные массы эквивалентов металла и оксида. Назвать металл.
 
| РЕШЕНИЕ:
По закону эквивалентов
nэк (окс) = nэк(О2)
 Мэк(Окс) =
Мэк(Окс) = 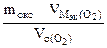
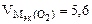 л/моль л/моль
|
По объединенному уравнению газового состояния
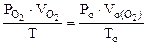
где: ТО = 273 К, РО = 760 мм рт.ст.
Определяем 
 =
= 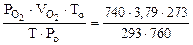 = 3,44 л.
= 3,44 л.
Мэк(Окс) = 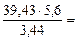 64,2 г/моль
64,2 г/моль
Мэк(Окс) = Мэк(Ме) + Мэк(О)
Мэк(Ме) = Мэк(Окс) - Мэк(О)
Мэк(Ме) = 64,2 – 8 = 56,2 г/моль
ММе = Мэк(Ме) ∙ В. Если В = 1, то МЭ = 56,2 г/моль. Такого металла в таблице Д.И. Менделеева нет. Если В = 2, то ММе = 112,4 г/моль. Следовательно, металл - кадмий
Ответ: Мэк(Окс) = 64,2 г/моль
Мэк(Ме) = 56,2 г/моль
Ме – Cd
3. Написать уравнения реакций взаимодействия гидроксида железа (III) с хлороводородной кислотой с образованием:
а) хлорида дигидроксожелеза (III);
б) хлорида гидроксожелеза (III);
в) хлорида железа (III).
В каждой реакции вычислить эквивалентную массу гидроксида железа (III). В одной из реакций определить массу гидроксида железа (III), необходимую для взаимодействия с 3,65 г хлороводородной кислоты.

| РЕШЕНИЕ:
Мэк (основ.) = 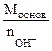 , г/моль , г/моль
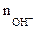 - число гидроксогрупп,
замещенных в данной реакции на кислотный остаток.
По реакциям а), б) и в):
Определяем - число гидроксогрупп,
замещенных в данной реакции на кислотный остаток.
По реакциям а), б) и в):
Определяем 
|
а) HCl + Fe(OH)3 = Fe(OH)2Cl + H2O
 =
=  = 106,8 г/моль
= 106,8 г/моль
б) 2HCl + Fe(OH)3 = FeOHCl2 + 2H2O
Mэк[Fe(OH)3] = 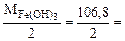 53,4 г/моль
53,4 г/моль
в) 3HCl + Fe(OH)3 = FeCl3 + 3H2O
Mэк[Fe(OH)3] =  35,6 г/моль.
35,6 г/моль.
По закону эквивалентов:
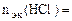
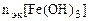 , т.е.
, т.е. 
Откуда по реакции (в)

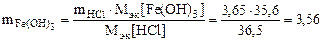 г,
г,
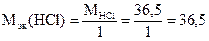 г/моль
г/моль
где Mэк[Fe(OH)3] = 35,6 г,моль
Ответ:а)Mэк[Fe(OH)3] = 106,8 г/моль
б) Mэк[Fe(OH)3] = 53,4 г/моль
в) Mэк[Fe(OH)3] = 35,6 г/моль.
 г.
г.
 3. СПОСОБЫ ВЫРАЖЕНИЯ СОСТАВА РАСТВОРА
3. СПОСОБЫ ВЫРАЖЕНИЯ СОСТАВА РАСТВОРА
УРОВЕНЬ A
1. Реакция с участием FeCl3 протекает по уравнению: FeCl3 + 2NaOH = Fe(OH)2Cl + 2NaCl. Определить массу хлорида железа (III), содержащуюся в 200 см3 0,1 н раствора хлорида железа (III).

| РЕШЕНИЕ
Молярную концентрацию эквивалентов FeCl3 определяем по формуле
Сэк(FeCl3) =  , моль/л
откуда , моль/л
откуда

|
Мэк(FeCl3) = 
 , г/моль
, г/моль
где 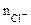 - число ионов Сl-, замещенных в данной реакции на ОН-;
- число ионов Сl-, замещенных в данной реакции на ОН-;
Мэк(FeCl3) -молярная масса эквивалентов хлорида железа (III), г/моль,
 - молярная масса хлорида железа (III), г/моль
- молярная масса хлорида железа (III), г/моль
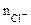 = 2, так как в данной реакции два иона хлора замещаются на 2 иона ОН-
= 2, так как в данной реакции два иона хлора замещаются на 2 иона ОН-
Тогда 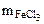 = 0,1· 200 · 10-3 · 81,25 = 1,625 г
= 0,1· 200 · 10-3 · 81,25 = 1,625 г
где Мэк(FeCl3) = 
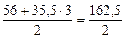 = 81,25 г/моль.
= 81,25 г/моль.
где 10-3 – пересчет см3 в л.
Ответ: 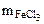 = 1,625 г.
= 1,625 г.
2. Сколько граммов хлорида магния потребуется для приготовления 800 см3 25%-ного водного раствора плотностью 1,2 г/см3?
Дано:
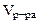 = 800 cм3 = 800 cм3
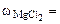 25% мас. 25% мас.
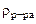 = 1,2 г/см3 = 1,2 г/см3
| РЕШЕНИЕ:
Массовая доля MgCl2 равна:


|
 ? ?
|
mр-ра = Vр-ра·ρр-ра = 800·1,2 = 960 г.
Откуда: 
Ответ: 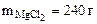
3. Определить массу сульфата меди (II), содержащуюся в 800 см3 0,3 М водного раствора сульфата меди (II).
Дано:
Vр-ра = 800 см3
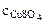 = 0,3 М = 0,3 М
| РЕШЕНИЕ:
Молярную концентрацию СuSO4 определяем по формуле:
 моль/л моль/л
|

|
где:
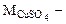 молярная масса сульфата меди (II), г/моль.
молярная масса сульфата меди (II), г/моль.
 = 64 + 32 + 64 = 160 г/моль
= 64 + 32 + 64 = 160 г/моль
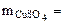
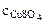 ·
·  ·
· 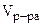 = 0,3·160·800·10-3 = 38,4 г
= 0,3·160·800·10-3 = 38,4 г
где 10-3 - пересчет см3 в л.
Ответ: 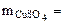 38,4 г.
38,4 г.





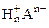
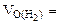 2,24 л
Мэк(Окс.) -?
Мэк(Ме) -? Ме -?
2,24 л
Мэк(Окс.) -?
Мэк(Ме) -? Ме -?
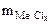 = 0,494г
= 0,494г
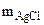 = 0,95 г
Mэк(Me) -?
Me -?
= 0,95 г
Mэк(Me) -?
Me -?
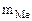 =16,25 г
В = 2
=16,25 г
В = 2
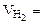 6,52 л
Т = 298 К
Робщ = 730 мм рт.ст
6,52 л
Т = 298 К
Робщ = 730 мм рт.ст
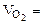 3,79 л
Т = 293 К
3,79 л
Т = 293 К
 = 740 мм рт.ст.
Мэк(Окс) -?
Мэк(Ме) -?
Ме -?
= 740 мм рт.ст.
Мэк(Окс) -?
Мэк(Ме) -?
Ме -?
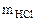 = 3,65 г
Fe(OH)3
Mэк[Fe(OH)3] -?
= 3,65 г
Fe(OH)3
Mэк[Fe(OH)3] -?

 -?
-?

