ѕример 17. ¬ычислить окислительно-восстановительный потенциал, если к 15,0 мл 0,20 ћ раствора KMnO4 добавили 50,0 мл 0,10 ћ раствора Na2SO3 при рЌ = 1.
–ешение. ѕри смешении растворов протекает реакци€ (см. пример 16). –ассчитаем количество вещества эквивалента в обоих растворах до сливани€:
 n (
n ( KMnO4) = 15,0 ∙ 10Ц3 ∙ 1,00 = 15,0 ∙ 10Ц3 моль экв
KMnO4) = 15,0 ∙ 10Ц3 ∙ 1,00 = 15,0 ∙ 10Ц3 моль экв
 n (
n (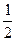 Na2SO3) = 50,0 ∙ 10Ц3 ∙ 0,20 = 10,0 ∙ 10Ц3 моль экв.
Na2SO3) = 50,0 ∙ 10Ц3 ∙ 0,20 = 10,0 ∙ 10Ц3 моль экв.
—ледовательно, KMnO4 вз€т в избытке. ѕосле протекани€ реакции в растворе будут содержатьс€ продукты реакции и остаток KMnO4. «начит, величина потенциала будет определ€тьс€ присутствием окислительно-восстановительной пары MnO4Ц/Mn2+. –ассчитаем количества этих ионов:
n (
 ) = 15,0 ∙ 10Ц3 Ц 10,0 ∙ 10Ц3 = 5,0 ∙ 10Ц3моль экв Þ
) = 15,0 ∙ 10Ц3 Ц 10,0 ∙ 10Ц3 = 5,0 ∙ 10Ц3моль экв Þ
n ( ) = 1,0 ∙ 10Ц3моль
) = 1,0 ∙ 10Ц3моль
n ( Mn2+) = 10,0 ∙ 10Ц3 моль экв Þ n (Mn2+) = 2,0 ∙ 10Ц3моль.
Mn2+) = 10,0 ∙ 10Ц3 моль экв Þ n (Mn2+) = 2,0 ∙ 10Ц3моль.
ќбъем раствора после смешени€ составит
15,0 + 50,0 = 65,0 мл = 65 ∙ 10Ц3 л.
–ассчитаем мол€рные концентрации ионов:
— ( ) =1,0 ∙ 10Ц3/ 65 ∙ 10Ц3 = 0,0154 моль/л
) =1,0 ∙ 10Ц3/ 65 ∙ 10Ц3 = 0,0154 моль/л
— (Mn2+) =2,0 ∙ 10Ц3/ 65 ∙ 10Ц3 = 0,0308 моль/л
C (H+) = 10Ц pH = 10Ц1 = 0,1 моль/л.
–ассчитаем окислительно-восстановительный потенциал раствора по уравнению Ќернста:
E =  + 0,059 / 5 ∙ lg (
+ 0,059 / 5 ∙ lg ( ) =
) =
= 1,51 + 0,059 / 5 ∙ lg(0,0154 ∙ (0,1)8/ 0,0308) = 1,49 ¬.
–асчет значени€ ≈ при протекании конкурирующих реакций осаждени€
ѕример 18. ¬ычислить окислительно-восстановительный потенциал пары  /Hg в присутствии ионов ClЦ, прин€в концентрацию ионов ClЦ равной 0,1 моль/л.
/Hg в присутствии ионов ClЦ, прин€в концентрацию ионов ClЦ равной 0,1 моль/л.
–ешение. ¬ присутствии ионов ClЦ в растворе образуетс€ малорастворимое соединение Hg2Cl2. ѕолуреакцию
Hg2Cl2 + 2 e = 2Hg + 2ClЦ
можно представить как сочетание реакций окислени€-восстановлени€ и осаждени€:
 + 2 e = 2Hg (4.2)
+ 2 e = 2Hg (4.2)
 + 2ClЦ = Hg2Cl2
+ 2ClЦ = Hg2Cl2
Ќайдем по справочнику [1; 2, с. 24, 49] необходимые константы:
 = 0,792 ¬
= 0,792 ¬
 = 1,3∙10Ц18
= 1,3∙10Ц18
»оны ртути (I) по€вл€ютс€ в растворе только за счет растворимости Hg2Cl2. Ќайдем их равновесную концентрацию из величины произведени€ растворимости и заданной концентрации хлоридов:
[  ] =
] =  / [ClЦ]2 = 1,3 ∙ 10Ц18/(0,1)2 =1,3 ∙ 10Ц16 моль/л
/ [ClЦ]2 = 1,3 ∙ 10Ц18/(0,1)2 =1,3 ∙ 10Ц16 моль/л
«апишем уравнение Ќернста дл€ полуреакции (4.2) и подставим имеющиес€ данные:

–асчет значени€ ≈ при протекании конкурирующих реакций комплексообразовани€
ѕример 19. ¬ычислить окислительно-восстановительный потенциал пары Cd2+/Cd в растворе, полученном смешением 40 мл 0,2 моль/л CdCl2 и 60 мл 4 моль/л KCN.
–ешение. ќкислительно-восстановительный потенциал пары Cd2+/Cd рассчитываетс€ по уравнению Ќернста:
 =
=  + 0,059 / 2 ∙ lg [Cd2+].
+ 0,059 / 2 ∙ lg [Cd2+].
–ассчитаем концентрации веществ после смешени€:


ѕосле смешени€ протекает реакци€ комплексообразовани€
Cd2+ + 4CNЦ ↔ 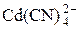
–авновесна€ концентраци€ ионов Cd2+ определ€етс€ процессом комплексообразовани€, протекающим в присутствии избытка CNЦ. –авновесие образовани€ комплекса 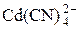 характеризуетс€ константой устойчивости:
характеризуетс€ константой устойчивости:
|
|
|
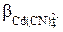 =
=  = 1,29 ∙ 1017
= 1,29 ∙ 1017
ѕри значительном избытке лиганда и достаточно большом значении константы устойчивости можно прин€ть, что
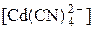 =
= 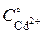 = 0,08 моль/л
= 0,08 моль/л
[CNЦ] = 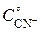 Ц 4 ∙
Ц 4 ∙ 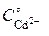 = 2,4 Ц 4 ∙ 0,08 = 2,08 моль/л
= 2,4 Ц 4 ∙ 0,08 = 2,08 моль/л
ѕодставим эти данные в выражение дл€ константы устойчивости, выразим равновесную концентрацию ионов Cd2+ и рассчитаем ее:

Ќайдем по справочнику  = Ц 0,402 ¬.
= Ц 0,402 ¬.
ќтсюда
 = Ц 0,402 + 0,059/2 ∙ lg 3,31 ∙ 10Ц20 = Ц 0,977 ¬.
= Ц 0,402 + 0,059/2 ∙ lg 3,31 ∙ 10Ц20 = Ц 0,977 ¬.
ќћѕЋ≈ —ќќЅ–ј«ќ¬јЌ»≈
ќсновные пон€ти€
–еакции комплексообразовани€ Ц это реакции с переносом электронных пар и образованием св€зей по донорно-акцепторному механизму. ¬ них участвуют металл-комплексообразователь (центральный атом, ÷ј) и лиганды (L). ¬ результате протекани€ реакции образуютс€ комплексные соединени€ (комплексы).
омплекс Ц это сложна€ частица, состо€ща€ из составных частей, способных к автономному существованию.
—остав комплекса:

¬ состав лиганда вход€т донорные атомы Ц атомы, которые св€зывают лиганд с центральным атомом, например, атомы O, N, S и др.
омплексообразователь характеризуют координационным числом ( „), а лиганд Ц дентатностью.
оординационное число центрального атома Ц это число мест во внутренней сфере комплекса, которые могут быть зан€ты лигандами.
ƒентатность лиганда Ц это число донорных атомов лиганда, которые образуют св€зи с ÷ј.






