1. Раствор сильного основания
В растворе сильного основания протолитическое равновесие полностью смещено вправо:
B + H2O ® BH+ + OH–
При Сосн. > 10–6 М автопротолизом воды можно пренебречь. Тогда равновесная концентрация гидроксид-ионов будет равна общей концентрации однокислотного основания:
[ОH–] = С осн. Þ
рОН = – lg[ОH–] = – lg С осн. Þ
рН = 14 – рОН = 14 + lg С осн. (3.8)
Если Скисл ≤ 10–6 М, то надо учесть автопротолиз воды, за счет которого в растворе тоже появляются гидроксид-ионы. В этом случае расчет равновесной концентрации ионов ОH– проводят по формуле:
 (3.9)
(3.9)
2. Раствор слабого основания
В растворе слабого основания устанавливается равновесие, которое смещено влево и характеризуется константой основности:
В+Н2О ↔ ВН++ОН–
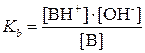
Если выполняются условия:
С осн. > 10–4 М

α < 5 %,
то можно пользоваться приближенными формулами для расчета рН, сделав допущение, что при указанных условиях равновесная концентрация недиссоциированного основания примерно равна его общей концентрации в растворе:
[B] ≈ Сосн.
Из уравнения реакции видно, что
[BH+] = [OH–] Þ
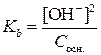
Отсюда выражаем равновесную концентрацию гидроксид-ионов в растворе слабого основания:

После логарифмирования с обратным знаком получаем:
 Þ
Þ
 (3.10)
(3.10)
Если степень диссоциации основания α > 5 %, то расчетная формула получается более громоздкой:
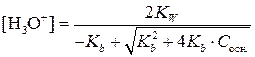 (3.11)
(3.11)
3. Раствор анионного слабого основания
Согласно протолитической теории, анионы слабых кислот являются анионными слабыми основаниями. Рассмотрим расчет значения рН на примере водного раствора ацетата натрия CH3COONa. Эта соль в растворе полностью диссоциирует:
CH3COONa ® CH3COO– + Na+
Ацетат-ион вступает в протолитическую реакцию с растворителем-амфолитом водой:
CH3COO– + H2O ↔ CH3COOH + OH–
В этой реакции уксусная кислота CH3COOH является молекулярной кислотой, а ион CH3COO– – сопряженным анионным основанием. Следовательно, для расчета рН в растворе CH3COONa выбираем формулу (3.10). В нее входит величина рKb, в нашем случае
рKb (CH3COO–), которой в таблицах нет. Для ее расчета используем табличное значение рKа (CH3COOH) и формулу (3.2):
рKb (CH3COO–) = 14 – рKа (CH3COOH)
Подставив это выражение в формулу (3.10), получим
 (3.12)
(3.12)
Пример расчета
Задание: рассчитать рН в 0,1 М растворе формиата калия HCOOK.
Решение: рассчитаем рН в 0,1 М растворе формиата калия HCOOK по формуле (3.12), подставив табличное значение константы ионизации муравьиной кислоты рKа (НСООН) = 3,75 и концентрацию соли в растворе:

4. Раствор многокислотного основания
В растворе многокислотного основания происходит ступенчатая диссоциация.
Например, в водном растворе этилендиамина H2NСH2–CH2NH2 образуется гидроксид этилендиаммония, который является двухкислотным основанием и диссоциирует по ступеням:
H2NСH2–CH2NH2 + 2H2O «НОH3NСH2–CH2NH3ОН
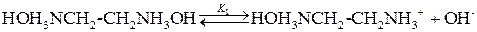

В результате образуется сложная многокомпонентная система, и расчет рН сильно усложняется. Поэтому полагают, то при выполнении условия
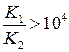 или в логарифмическом виде pK 2 – pK 1 > 4
или в логарифмическом виде pK 2 – pK 1 > 4
диссоциация основания по второй ступени подавляется, и рассчитывают значение рН как в растворе слабого однокислотного основания, используя формулы (3.10) или (3.11).






