як вже зазначалось, до другоњ анал≥тичноњ групи в≥днос€ть кат≥они Ag+, Hg22+, Pb2+.
≈лектронна будова цих йон≥в наступна:
Ag+ Ц [Kr]5s14d10; Hg22+ Ц [Xe]6s14f145d10; Pb2+ Ц [Xe]4f146p25d10.
√руповим реагентом на цю групу кат≥он≥в Ї розчин хлоридноњ кислоти з с(1/1HCl)=2,0моль/дм3.
ат≥они Ag+, Hg22+, Pb2+ при взаЇмод≥њ з хлорид-≥онами утворюють малорозчинн≥ у вод≥ ≥ в розведених кислотах осади:
Ag+ + Cl− → AgCl↓; Hg22+ + 2Cl− → Hg2Cl2↓; Pb22+ + 2Cl− → PbCl2↓;
” надлишку хлоридноњ кислоти утворен≥ осади можуть частково розчин€тись, переход€чи у розчинн≥ комплекси:
AgCl↓ + 2HCl → H2[AgCl3]; PbCl2↓ + HCl → H[PbCl3].
” кипл€ч≥й вод≥ розчинн≥сть PbCl2 зб≥льшуЇтьс€ втрич≥ у пор≥вн€нн≥ ≥з розчинн≥стю при 200—. «а цих же умов розчинн≥сть AgCl та Hg2Cl2 в≥дчутно анал≥тичними методами не зростаЇ. ÷€ особлив≥сть використовуЇтьс€ дл€ в≥дд≥ленн€ йон≥в Pb2+ в≥д Ag+ та Hg22+.
«а д≥њ розчину амон≥аку Hg2Cl2 перетворюЇтьс€ на димеркур≥йамон≥й хлорид, €кий швидко розкладаЇтьс€ на малорозчинний меркур≥й амон≥й хлорид та метал≥чну ртуть, €ка надаЇ осаду чорного забарвленн€:
Hg2Cl2 + 2NH4OH → [Hg2NH2]Cl↓ + NH4+ + ClЦ +2H2O;
[Hg2NH2]Cl → [HgNH2]Cl↓ + Hg.
ƒ≥Їю розчином амон≥аку на сум≥ш кат≥он≥в Ag+ та Hg22+ вдаЇтьс€ њх розд≥лити: йони Ag+ звТ€зуютьс€ в розчинний комплекс [јg(NH3)2]+, а йони Hg22+ Ц у нерозчинний комплекс [HgNH2]Cl.
4.2.1. –еакц≥њ ви€вленн€ кат≥он≥в Ag+
—пециф≥чних реакц≥й ви€вленн€ кат≥он≥в Ag+ немаЇ.
ƒосл≥д 1. ≤з загальних можливих реакц≥й можна зазначити д≥ю на кат≥они Ag+ розчинами луг≥в NaOH або KOH. як пром≥жний продукт спочатку утворюЇтьс€ б≥лий осад AgOH, €кий п≥д д≥Їю св≥тла розкладаЇтьс€ ≥ утворюЇтьс€ осад аргентум оксиду бурого забарвленн€:
Ag+ + OHЦ → AgOH↓;
2AgOH → Ag2O↓ + H2O.
ќсад Ag2O дуже добре розчин€Їтьс€ у розчин≥ амон≥аку:
Ag2O + 4NH4OH → 2[јg(NH3)2]ќЌ + 3H2O.
—л≥д зазначити, що молекул€рноњ сполуки NH4OH €к такоњ не
| ≥снуЇ. Ќасправд≥ це комплекс | 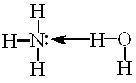
| , | €кий у водному |
середовищ≥ дисоц≥юЇ з утворенн€м йон≥в [NH4]+ та ќЌ−. ѕо сут≥, г≥дроксид-≥они поход€ть з води, з €коњ протон в≥дщеплюЇтьс€ ≥ звТ€зуЇтьс€ у м≥цний комплекс [NH4]+ з константою нест≥йкост≥ нест[NH4]+ =6Ј10Ц10. –≥вноважна концентрац≥€ йон≥в Ќ+ у систем≥
[NH4]+ 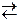 NH3 + H+ менша, н≥ж р≥вноважна концентрац≥€ г≥дроген-≥он≥в у систем≥ Ќ2ќ
NH3 + H+ менша, н≥ж р≥вноважна концентрац≥€ г≥дроген-≥он≥в у систем≥ Ќ2ќ 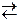 H+ + ќЌЦ, тому середовище водного розчину амон≥аку лужне.
H+ + ќЌЦ, тому середовище водного розчину амон≥аку лужне.
ƒосл≥д 2. ат≥они Ag+ з хромат-≥онами утворюють малорозчинний осад аргентум хромату цегельно-червоного кольору:
2Ag+ + CrO42Ц → Ag2CrO4↓.
÷ю реакц≥ю сл≥д проводити при рЌ=5,5-7,5.
” присутност≥ в розчин≥ амон≥аку осад не утворюЇтьс€ внасл≥док включенн€ йон≥в Ag+ в безбарвний водорозчинний комплекс [јg(NH3)2]+:
Ag2CrO4 + 4NH3ЈH2O → 2[јg(NH3)2]+ + CrO42Ц + 4H2O.
ќсад аргентум хромату не утворюЇтьс€ також у сильнокислому середовищ≥. ” сильнокислому середовищ≥ хромат переходить у дихромат Ag2Cr2O7, €кий хоч ≥ маЇ забарвленн€, але добре розчинний у вод≥.
|
|
|
якщо в досл≥джуваному середовищ≥ присутн≥ йони, що утворюють з CrO42Ц-≥онами також осади (наприклад, Pb2+, Ba2+ та ≥нш≥), то вони будуть заважати ви€вленню йон≥в Ag+ за ц≥Їю реакц≥Їю.
ƒосл≥д 3. √алоген≥д-≥они (Br−, I−) утворюють з кат≥онами Ag+ осади AgBr жовтуватого забарвленн€ та AgI жовтого забарвленн€:
Ag+ + ¬r− → AgBr↓;
Ag+ + I− → AgI↓.
јргентум йодид розчинний у надлишку реагенту внасл≥док комплексоутворенн€:
AgI↓ + I → [AgI2],
а також можливе розчиненн€ шл€хом колоњдоутворенн€:
{m(AgI)ЈnIЦ∙(nЦx)K+}−xK+.
4.2.2. –еакц≥њ ви€вленн€ кат≥он≥в Pb2+
ƒосл≥д 1. ” лужному середовищ≥ кат≥они Pb2+ утворюють малорозчинний у вод≥ плюмбум г≥дроксид б≥лого кольору:
Pb2+ + 2ќЌ− → Pb(ќЌ)2↓,
€кий розчинний €к у кислотах, так ≥ в концентрованих розчинах луг≥в:
Pb(ќЌ)2↓ + 2Ќ+ → Pb2+ + 2Ќ2ќ;
Pb(ќЌ)2↓ + 2Na+ + 2OHЦ → 2Na+ + [Pb(ќЌ)4]2Ц;
[Pb(ќЌ)4]  PbO22Ц + 2H2O.
PbO22Ц + 2H2O.
якщо дл€ розчиненн€ був вз€тий натр≥й г≥дроксид, то утворюЇтьс€ водорозчинний натр≥й плюмб≥т Na2PbO2.
ќтже, при додаванн≥ до розчину, що м≥стить йони Pb2+, надлишку лугу, осад Pb(ќЌ)2 ≥ не утворюЇтьс€.
ƒосл≥д 2. ѕри д≥њ сульфат-≥он≥в на йони Pb2+ утворюЇтьс€ малорозчинний PbSO4 (ƒ–(PbSO4)=1,6Ј10Ц8. Ќа ц≥й основ≥ йони Pb2+ в≥днос€ть також ≥ до третьоњ (сульфатноњ) анал≥тичноњ групи кат≥он≥в.
ѕри нагр≥ванн≥ осаду PbSO4 з розчинами луг≥в утворюютьс€ плюмб≥ти:
PbSO4 + 4OH− → PbO22Ц + 2H2O + SO42− .
ѕлюмбум сульфат розчин€Їтьс€ також у розчин≥ амон≥й ацетату (w=30%). ѕри цьому утворюЇтьс€ водорозчинний молекул€рний комплекс [Pb(CH3COO)2PbSO4]:
2PbSO4 + 2CH3COO− → [Pb(CH3COO)2PbSO4] + SO42Ц.
–озчинн≥сть осаду PbSO4 п≥двищуЇтьс€ у середовищ≥ сильних кислот, так €к при цьому йони SO42Ц, €к≥ утворюютьс€ за вищенаведеною реакц≥Їю, звТ€зуютьс€ в ан≥они ЌSO4Ц ≥ р≥вновага зм≥щуЇтьс€ праворуч: Ќ+ + SO42Ц 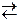 ЌSO4−.
ЌSO4−.
ƒосл≥д 3. ал≥й хромат та кал≥й дихромат утворюють з кат≥онами Pb2+ малорозчинний плюмбум хромат жовтого кольору:
Pb2+ + CrO42Ц → PbCrO4↓.
ѕлюмбум хромат розчинний в розчинах г≥дроксид≥в:
PbCrO4 + 4NaOH → Na2[Pb(OH)4] + Na2CrO4;
PbCrO4 + 4OHЦ → [Pb(OH)4]2Ц + CrO42Ц,
але нерозчинний в оцтов≥й кислот≥.
ѕри взаЇмод≥њ йон≥в Pb2+ з водним розчином амон≥аку утворюютьс€ б≥л≥ осади Pb(OH)2 або Pb(OH)јn (јn Ц однозар€дний ан≥он), €к≥ не розчин€ютьс€ у надлишку реагенту через в≥дсутн≥сть схильност≥ ѕлюмбуму до утворенн€ комплексних амон≥акат≥в.
ƒосл≥д 4. —пециф≥чного реакц≥Їю на кат≥они Pb2+ Ї взаЇмод≥њ њх з йодид-≥онами:
Pb2+ + 2I− → PbI2↓.
ѕри цьому утворюютьс€ осад оранжево-жовтого забарвленн€.
ѕри нагр≥ванн€ осад PbI2 у присутност≥ дек≥лькох крапель оцтовоњ кислоти (с(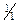 CH3COOЌ)=2моль/дм3) розчин€Їтьс€, а при охолодженн≥ знову утворюЇтьс€ у вигл€д≥ блискучих золотистих кристал≥в.
CH3COOЌ)=2моль/дм3) розчин€Їтьс€, а при охолодженн≥ знову утворюЇтьс€ у вигл€д≥ блискучих золотистих кристал≥в.
–еакц≥ю утворенн€ PbI2 сл≥д проводити у кислому середовищ≥ при рЌ=3-5.
” надлишку реагенту осад PbI2 розчин€Їтьс€. ѕри цьому утворюЇтьс€ водорозчинний комплекс [PbI4]2Ц:
PbI2 + 2KI → K2[PbI4].
–еакц≥€ йон≥в Pb2+ з йодид-≥онами дозвол€Ї ви€вити њх у присутност≥ кат≥он≥в вс≥х анал≥тичних груп.
ƒосл≥д 5. ƒуже чутливою реакц≥Їю на йони Pb2+ Ї њх взаЇмод≥€ з д≥т≥зоном, при цьому утворюЇтьс€ хелатний комплекс плюмбум д≥т≥зонату, €кий маЇ цегельно-червоне забарвленн€:
|
|
|
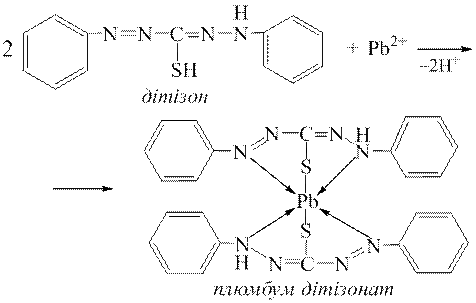
¬изначенню йон≥в Pb2+ за реакц≥Їю з д≥т≥зоном заважають йони де€ких важких метал≥в (Fe3+, Cu2+, Ni2+, Zn2+ та ≥н.), €к≥ утворюють з д≥т≥зоном забарвлен≥ хелатн≥ комплекси.
ƒосл≥д 6. Ћетк≥ сполуки ѕлюмбуму забарвлюють полумТ€ у св≥тло-блакитний кол≥р.
4.2.3. –еакц≥њ ви€вленн€ кат≥он≥в [Ќg2]2+
” комплексному меркур≥й(≤)-≥он≥ [ЦHgЦHgЦ]2+ ступ≥нь окисненн€ ћеркур≥ю становить 1+.
ƒосл≥д 1. ѕри д≥њ групового реагенту кат≥они [Ќg2]2+ утворюють дуже малорозчинний Hg2Cl2 б≥лого кольору, €кий чорн≥Ї при додаванн≥ водного розчину амон≥аку з утворенн€м осаду
[NH2Hg]Cl + Hg. ÷ей зм≥шаний осад розчин€Їтьс€ повн≥стю т≥льки при кипТ€т≥нн≥ з концентрованою н≥тратною кислотою:
3[NH2Hg]Cl + 3Hg + 14H+ + 14NO3−→ 6Hg2+ + 12NO3− + 3NH4+ + 3Cl− + 4H2O +2NO.
ƒосл≥д 2. ” лужному середовищ≥ йони [Ќg2]2+ утворюють осад меркур≥й(≤) оксиду, €кий маЇ чорне забарвленн€:
[Ќg2]2+ + 2OHЦ → Hg2O↓ + H2O.
Hg2O розкладаЇтьс€ на HgO та Hg.
ƒосл≥д 3. « водним розчином амон≥аку меркур≥й(≤)-≥они утворюють малорозчинний комплекс димеркур≥йамон≥й хлориду [Ќg2NH2]Cl.
ƒосл≥д 4. « хромат-≥онами йони [Ќg2]2+ утворюють осад
| червоного кольору меркур≥й(≤) хромату | 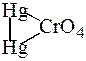
| , €кий нерозчинний |
в розчинах луг≥в та погано розчинний у розведен≥й н≥тратн≥й кислот≥.
ƒосл≥д 5. …они [Ќg2]2+ можна ви€вити в≥дновлюючи њх до метал≥чноњ ртут≥ досить сильним в≥дновником, наприклад Sn2+ у вигл€д≥ станум(≤≤) хлориду. –озчин SnCl2 маЇ бути
св≥жоприготовлений з на€вн≥стю у розчин≥ хлоридноњ кислоти та гранул олова на дн≥ посудини.
—початку утворюЇтьс€ осад б≥лого кольору Hg2Cl2:
[Ќg2]2+ + 2—lЦ → Hg2Cl2↓,
€кий дал≥ взаЇмод≥Ї з йонами Sn2+:
Hg2Cl2 + Sn2+ → 2Hg↓ + Sn4+ + 2—lЦ.
¬≥д метал≥чноњ ртут≥ вм≥ст проб≥рки набуваЇ чорного забарвленн€.
ƒосл≥д 6. ¬и€вити йони [Ќg2]2+ можна також за допомогою реакц≥њ з метал≥чною м≥ддю. ћ≥дну пластинку зачищають наждачним папером до блискучого стану, а пот≥м на очищену поверхню нанос€ть краплю розчину Hg2(NO3)2. „ерез дек≥лька хвилин спостер≥гають утворенн€ с≥роњ пл€ми метал≥чноњ ртут≥:
Hg2(NO3)2. + —u → —u(NO3)2. + 2Hg↓.
якщо утворену пл€му протерти ф≥льтрувальним папером, то пл€ма стане дзеркально блискучою. ѕроведенню ц≥Їњ реакц≥њ заважають сильн≥ окисники.
ƒосл≥д 7. ат≥они [Ќg2]2+ з дифен≥лкарбазоном утворюють сполуку синього забарвленн€:

ƒл€ проведенн€ ц≥Їњ реакц≥њ на скл€ну плат≥вку нанос€ть краплю розчину сол≥ Hg2(NO3)2, додають краплю розчину розведеноњ (w=2%) н≥тратноњ кислоти та краплю розчину дифен≥лкарбазону. «абарвленн€ стаЇ син≥м або ф≥олетовим.
“аке ж забарвленн€ дають йони Hg2+ та CrO42Ц, тому перед визначенн€м йон≥в [Ќg2]2+ йони, що заважають, мають бути видален≥.
” нейтральних та оцтовокислих розчинах кат≥они Cu2+, Fe2+, Fe3+, Cr3+, Co2+, та ≥нш≥ теж утворюють з дифен≥лкарбазоном забарвлен≥ сполуки, а тому заважають ви€вленню йон≥в [Ќg2]2+ за ц≥Їю реакц≥Їю.
ƒосл≥д 8. ѕри взаЇмод≥њ кат≥он≥в [Ќg2]2+ з кал≥й йодидом утворюЇтьс€ дуже малорозчинний Hg2I2 жовто-зеленого забарвленн€:
[Ќg2]2+ + 2IЦ → Hg2I2↓,
€кий розчин€Їтьс€ у великому надлишку реактиву з утворенн€м комплексу кал≥й тетрайодмеркур≥ю та метал≥чноњ ртут≥:
Hg2I2 + 2 I → 2[Ќg≤4] + Hg↓.






