Задача 1. Произведение растворимости сульфата бария равно 1·10-10. Вычислить массу сульфата бария в 5 л насыщенного раствора.
Так как в насыщенном, но очень разбавленном растворе практически все молекулы диссоциируют на ионы BaSO4 ⇄ Ba2+ + SO 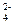 ,
,
то [BaSO4] = [Ba2+] = [SO 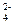 ].
].
По условию задачи ПР(BaSO4) = [Ba2+] [SO 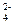 ] = 1·10-10, следовательно, ПР(BaSO4) = [BaSO4]2, откуда [BaSO4] =
] = 1·10-10, следовательно, ПР(BaSO4) = [BaSO4]2, откуда [BaSO4] =  =
=  = 10-5 моль/л.
= 10-5 моль/л.
Найдем число молей (ν) в 5 л: ν = 5·10-5 моль.
Чтобы определить массу сульфата бария (m), нужно величину ν умножить на молярную массу BaSO4:
m = ν M (BaSO4) = 5·10-5 ·233 = 1,165·10-2 г = 11,65 мг.
Задача 2. Растворимость иодида серебра AgI при 250С равна 1,22·10-8 моль/л. Вычислить произведение растворимости AgI.
AgI ⇄ Ag+ + I-
[Ag+] = [I-] = [AgI] = 1,22·10-8 моль/л.
ПР(AgI) = [Ag+] [I-] = (1,22·10-8)2 = 1,5·10-16.
Зная произведение растворимости, можно определить, образуется ли осадок при сливании двух растворов известной концентрации. Условие образования осадка: осадок образуется в том случае, если произведение концентраций ионов в растворе, полученном после смешения двух растворов, больше или равно произведению растворимости.
Задача 3. Смешаны равные объемы 0,02 М растворов хлорида кальция и сульфата натрия. Образуется ли осадок сульфата кальция? ПР(CaSO4) = 1,3·10-4.
Хлорид кальция и сульфат натрия - сильные электролиты, поэтому концентрации катионов кальция и сульфат-анионов равны молярным концентрациям солей: [Ca2+]1 = [CaCl2] = 0,02 моль/л и [SO 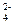 ]1 = [Na2SO4] = 0,02 моль/л.
]1 = [Na2SO4] = 0,02 моль/л.
При смешении равных объемов общий объем увеличился вдвое. Концентрация ионов [Ca2+]2 и [SO 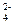 ]2 уменьшается вдвое по сравнению с исходными концентрациями: [Ca2+]2 = 0,5·0,02 = 10-2 моль/л,
]2 уменьшается вдвое по сравнению с исходными концентрациями: [Ca2+]2 = 0,5·0,02 = 10-2 моль/л,
[SO 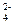 ]2 = 0,5·0,02 = 10-2 моль/л.
]2 = 0,5·0,02 = 10-2 моль/л.
Произведение концентраций этих ионов в растворе после смешения [Ca2+][SO 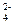 ] = 10-2·10-2 = 10-4, что меньше произведения растворимости: ПР(CaSO4) = 1,3·10-4. Следовательно, раствор не будет насыщенным и осадок не образуется.
] = 10-2·10-2 = 10-4, что меньше произведения растворимости: ПР(CaSO4) = 1,3·10-4. Следовательно, раствор не будет насыщенным и осадок не образуется.






