Пример. Электролиз водного раствора нитрата натрия с инертными электродами:
NaNO3 = Na + + NO3–
 (-) Kатод Na+, H2O (+) Aнод NO3–, H2O
(-) Kатод Na+, H2O (+) Aнод NO3–, H2O
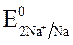 = - 2,71 B;
= - 2,71 B; 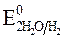 = -1 B. Нитрат-ионы не разряжаются, т.к.
= -1 B. Нитрат-ионы не разряжаются, т.к.
Так как 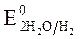 >
> 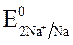 азот находится в высшей ст. ок.
азот находится в высшей ст. ок.
происходит восстановление воды: 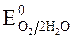 » 1,8 B.
» 1,8 B.
2H2O +2ē = H 2+ 2 OH - 2H2O - 4ē = O2 + 4 H +.
Среда щелочная Среда кислая.
ВОПРОСЫ ДЛЯ САМОКОНТРОЛЯ
Тема 1: «Строение атома»
Задание 1. Напишите электронные формулы атомов и ионов. Укажите валентные электроны атомов.
Задание 2. Распределите по квантовым ячейкам валентные электроны и определите какой это элемент.
Задание 3. Укажите значение главного и орбитального (побочного) квантовых чисел для подуровней
| № Варианта | Задание 1. | Задание 2. | Задание 3 |
| 1. | Ag, Ba2+ | 2s22p2 | 2p |
| 2. | As, Ti4+ | 2s22p6 | 4f |
| 3. | Sr, In3+ | 3s23p3 | 3d |
| 4. | Xe, Br- | 3s2 | 3s |
| 5. | Cs, Ga3+ | 2s22p1 | 4p |
| 6. | F, Mg2+ | 3s23p1 | 5d |
| 7. | S, Li+ | 6s2 | 4s |
| 8. | N, S2- | 5s25p3 | 3p |
| 9. | Ne, Al3+ | 5s25p2 | 4d |
| № Варианта | Задание 1. | Задание 2. | Задание 3 |
| 10. | Si, Na+ | 4s23d2 | 5p |
| 11. | O, I - | 5s24d2 | 5s |
| 12. | P, F- | 4s13d10 | 5f |
| 13. | Mg, Ar | 6s25d2 | 2s |
| 14. | K, S2- | 6s25d5 | 1s |
| 15. | Ca, Cl- | 6s26p3 | 5d |
| 16. | Al, Te2- | 5s25p1 | 1s |
| 17. | C, K+ | 6s25d4 | 6d |
| 18. | Se, Be2+ | 5s25p6 | 5f |
| 19. | Mn, Se2- | 6s15d10 | 7s |
| 20. | Cl, Si4+ | 5s14d10 | 4p |
| 21. | Si, Ge4+ | 5s25p4 | 5s |
| 22. | Na, B3+ | 6s26p6 | 6s |
| 23. | Zn, Hg2+ | 3s23p5 | 2p |
| 24. | Ar, Zn2+ | 2s22p2 | 3d |
| 25. | He, Hf | 3s23p3 | 2s |
| 26. | B, Y3+ | 3s23p1 | 3p |
| 27. | Au, P5+ | 3s23p6 | 4s |
| 28. | Cd, Br- | 2s22p4 | 4f |
| 29. | Ne, Sn2+ | 3s23p5 | 1s |
| 30. | At, Cr3+ | 4s1 | 5d |
Пример решения и оформления задания раздела 1.
1. Пример. Напишите электронные формулы атомов и ионов. Подчеркните валентные электроны.
4Ве: 1s2 2s2
9F–: 1s22s22p6.
2. Пример. Распределите по квантовым ячейкам валентные электроны и определите какой это элемент.
1s22s22p4 - это кислород 8O (8-ой порядковый номер)

3. Пример. Укажите значение главного и орбитального (побочного) квантовых чисел для подуровней.
6d: n = 6
l = 2
Тема 2 «Закономерности химических процессов»
2.1. «Термохимия»
Задание 1. Приведите значение теплового эффекта реакции при стандартных условиях, укажите – это экзо- или эндотермическая реакция (см. таблицу «термодинамических величин» в приложении).
Задание 2. Определите температуру, при которой устанавливается химическое равновесие реакции (см. таблицу «термодинамических величин» в приложении).
Задание 3. Сделайте вывод о возможности протекания данной реакции при стандартных условиях и при температуре 1500К.
| № варианта | Реакция |
| 1. | 2SO2(г)+O2(г)Û2SO3(г) |
| 2. | 4NH3(г)+5O2(г)Û4NO(г)+6H2O(ж) |
| 3. | CO2(г)+C(графит)Û2CO2(г) |
| 4. | 4NO(г)Û2N2O(г)+O2(г) |
| 5. | CH4(г)ÛC(графит)+2H2(г) |
| 6. | CaO(тв)+H2(г)ÛCa(тв)+H2O(г) |
| 7. | 4HCl(г)+O2(г)Û2H2O(г)+2Cl2(г) |
| 8. | N2(г)+3H2(г)Û2NH3(г) |
| 9. | 2HNO3(г)ÛH2(г)+N2(г)+3O2(г) |
| 10. | 2N2(г)+O2(г)Û2N2O(г) |
| 11. | C(графит)+H2O(г)ÛCO(г)+H2(г) |
| 12. | CO(г)+H2O(г)ÛCO2(г)+H2(г) |
| 13. | 2Ag2O(тв)Û4Ag(тв)+O2(г) |
| 14. | SiF4(г)ÛSi(тв)+2F2(г) |
| 15. | CO(г)+O2(г)Û2CO2(г) |
| 16. | 3S(тв)+2H2O(ж)ÛSO2(г)+2H2S(г) |
| 17. | SO2(г)+NO2(г)ÛSO3(г)+NO(г) |
| 18. | C(графит)+2F2(г)ÛCF4(г) |
| 19. | 2H2S(г)+O2(г)Û2S(тв)+2H2O(г) |
| 20. | Cl2(г)+2O2(г)Û2ClO2(г) |
| 21. | 2H2(г)+O2(г)Û2H2O(г) |
| № варианта | Реакция |
| 22. | 4NO2(г)Û2NO(г)+O2(г) |
| 23. | O2(г)+2F2(г)Û2OF2(г) |
| 24. | H2S(г)+Cl2(г)Û2HCl(г)+S(тв) |
| 25. | CO(г)+H2(г)ÛC(графит) + H2O(г) |
| 26. | SO2(г)+2H2S(г)Û3S(тв)+2H2O(ж) |
| 27. | С(алмаз)+H2O(г)ÛCO(г)+H2(г) |
| 28. | 2N2O(г)+O2(г)Û4NO(г) |
| 29. | Si(тв)+3F2(г)ÛSiF6(г) |
| 30. | CO(г)+Cl2(г)ÛCOCl2(г) |






