2.4. ¬ указанном комплексном соединении определите степени окислени€ всех составл€ющих, укажите комплексообразователь, лиганды, ионы внешней и внутренней сфер и координационное число, зар€д комплексообразовател€.
«апишите уравнение диссоциации данного комплексного соединени€. Ќазовите это соединение.
“ а б л и ц а 2.5
| ¬ариант | —оединение | ¬ариант | —оединение |
| [Zn(NH3)4]Cl2 | 1' | [Co(H2O)(NH3)4(CN)]Br2 | |
| [Al(H2O)6]Cl3 | 2' | [Co(NH3)5(SO4)]NO3 | |
| K2[BeF4] | 3' | [Pd(NH3)3Cl]Cl | |
| K[Al(OH)4] | 4' | (NH4)3[RhCl6] | |
| K2[Be(SO4)2] | 5' | K2[Co(NH3)2(NO2)4] | |
| [Pt(NH3)2Cl2] | 6' | K2[Pt(OH)5Cl] | |
| [Co(NH3)5Cl]Cl2 | 7' | K2[Cu(CN)4] | |
| [Cr(H2O)5Cl]SO4 | 8' | [Cr(H2O)4(PO4)] | |
| [Cr(H2O)4Cl2]Cl | 9' | [Cu(NH3)2(SCN)2] | |
| [Pt(NH3)3Cl]Cl | 10' | [Rh(NH3)3(NO2)3] | |
| [Co(NH3)5Br]SO4 | 11' | [Pt(NH3)2Cl4] | |
| Ba[Cr(NH3)2(SCN)4]2 | 12' | [Co(NH3)5(H2O)]Cl3 | |
| (NH4)2[Pt(OH)2Cl4] | 13' | [Ag(NH3)2]NO3 | |
| [Pd(H2O)(NH3)2Cl]Cl | 14' | K[Ag(CN)2] | |
| [Cu(NH3)4](NO3)2 | 15' | [Co(NH3)3(NO2)3] |
2.5. ќпределите степени окислени€ элементов в следующих сое-динени€х: K2MnO4, Ba(ClO3)2, F2O, Ca(NO3)2,H2SiF6, H2O2, Cr2(SO4)3.
2.6. —ероводород при обычной температуре Ц газ, а вода Ц жидкость. „ем можно объ€снить это различие в свойствах?
2.7. ¬ чем заключаетс€ сущность донорно-акцепторного меха-низма образовани€ химической св€зи? ѕриведите три примера соединений, св€зь в которых образована по этому механизму.
2.8. ƒайте характеристику водородной св€зи. ¬ каких случа€х возможно ее образование? ѕриведите примеры.
2.9. ѕриведите формулы четырех соединений, в состав которых вход€т ионы с электронной конфигурацией 1 s 22 s 22 p 63 s 23 p 6.
2.10. ѕочему существует ион NH 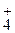 ?
?
2.11. —колько электронов и протонов содержат следующие молекулы и ионы: Ќ3ќ+, NCl3 ?
2.12. ќпределите степени окислени€ элементов в следующих соединени€х: CaCrO4, Sr(ClO)2, F2O,—а3(–ќ4)2, NH4NO3, Fe2(SO4)3.
2.13. ќпишите пространственное строение следующих молекул: AlCl3 и –Ќ3. ќбъ€сните причины их различи€.
2.14. ќпишите пространственное строение следующих молекул: Ќ2ќ, ¬еF2. ќбъ€сните причины их различи€.
2.15. ќпределите степени окислени€ элементов в следующих соединени€х: FeS, K3[Fe(CN)6], Fe2O3, Mn[PtF6], CH2Cl2, CH3COOH.
2.16. акую валентность может про€вл€ть сера в своих соединени€х? »зобразите структуру атома серы в нормальном и возбужденном состо€ни€х.
2.17. ќбъ€сните, почему максимальна€ валентность фосфора может быть равной п€ти.
2.18. аково взаимное расположение электронных облаков при sp 3-гибридизации? ѕриведите примеры соответствующих соедине-ний. акую пространственную конфигурацию могут иметь молекулы веществ с таким типом гибридизации?
2.19. ¬ чем причина различной пространственной структуры молекул BCl3 и NH3?
2.20. акую форму могут иметь трехатомные молекулы типа ј¬2? –ассмотрите на примерах —ќ2 и Ќ2ќ.
2.21. ќбъ€сните механизм образовани€ молекулы SiF4.
|
|
|
2.22. ак измен€етс€ прочность св€зи в р€ду: HFЦHClЦHBrЦHI? ”кажите причины этих изменений.
2.23. ќбъ€сните механизм образовани€ ковалентных св€зей в молекулах —Ќ4, NH3 и в ионе NH 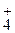 .
.
2.24. ќбъ€сните механизм образовани€ молекулы BF3 и иона BF 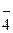 . акой атом или ион служит донором электронной пары при образовании иона BF
. акой атом или ион служит донором электронной пары при образовании иона BF 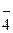 ?
?
2.25. ƒипольные моменты молекул BF3 и NF3 равны соответственно 0 и 0,2 D. ќбъ€сните причины непол€рности первой и пол€рности второй молекул.
2.26. акую ковалентную св€зь называют σ-св€зью и какую π-св€зью? ќтвет разберите на конкретных примерах.
2.27. аково взаимное расположение электронных облаков при sp 2-гибридизации? акую пространственную конфигурацию могут иметь молекулы веществ с таким типом гибридизации? ѕриведите примеры соответствующих соединений.
2.28. ¬ чем заключаетс€ sp -гибридизаци€ атомных орбиталей? ѕриведите примеры молекул, при образовании которых происходит sp -гибридизаци€ атомных орбиталей. акова структура этих молекул?
2.29. ѕриведите формулы четырех соединений, в состав которых вход€т ионы с электронной конфигурацией 1 s 22 s 22 p 6.
2.30. акие типы химических св€зей вам известны? акой тип св€зи в следующих соединени€х: HCl, Cl2, RbCl? ќтветы по€сните.
2.31. ћолекула PbCl2 углова€, а молекула HgCl2 линейна€. ќбъ€сните различную пространственную структуру этих молекул.
2.32. акие атомы или ионы называют донорами и акцепторами электронных пар? ѕриведите примеры.
2.33. акие элементы II периода могут быть донорами и акцепторами электронных пар? „ем это определ€етс€? ¬озможна ли донорна€ или акцепторна€ функци€ дл€ центрального атома в молекулах: а) BeF2; б) BF3; в) CF4; г) NH3; д) H2O; е) PCl5?
2.34. ѕриведите примеры молекул, которые содержат: а) только σ-св€зи; б) одну σ- и одну π-св€зь; в) две σ- и одну π-св€зь; г) две σ- и две π-св€зи; д) четыре σ- и две π-св€зи.
2.35. —колько σ- и π-св€зей содержат молекулы: а) SF6, CCl4; б) SO3, SO2; в) PCl5, POCl3; г) C2H4; C2H2; д) SO2Cl2, COCl2?
’»ћ»„≈— јя “≈–ћќƒ»Ќјћ» ј
–ешение типовых задач
ѕример 3.1. –еакци€ горени€ протекает по уравнению
—Ќ4 (г) + 2ќ2 (г) = —ќ2 (г) + 2Ќ2ќ (ж) + 890,31 кƒж
¬ычислите, сколько теплоты выделитс€ при сгорании 100 л (н.у.) углеводорода?
– е ш е н и е
¬ термохимическом уравнении дано значение стандартной энтальпии сгорани€ 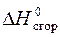 , то есть тепловой эффект реакции окислени€ 1 мол€ (22,4 л) газообразного метана (—Ќ4). 100 л газа в нормальных услови€х (н.у.) соответствуют
, то есть тепловой эффект реакции окислени€ 1 мол€ (22,4 л) газообразного метана (—Ќ4). 100 л газа в нормальных услови€х (н.у.) соответствуют 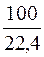 мол€м газа, следовательно, при сгорании 100 л газа выделитс€
мол€м газа, следовательно, при сгорании 100 л газа выделитс€ 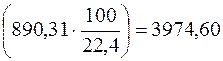 кƒж теплоты (зависимостью Δ Ќ от температуры пренебречь).
кƒж теплоты (зависимостью Δ Ќ от температуры пренебречь).
ѕример 3.2. ѕри соединении 27 г алюмини€ с кислородом выделилось 836,8 кƒж теплоты. ќпределите энтальпию образовани€ оксида алюмини€ (Al2O3).
– е ш е н и е
Ёнтальпией образовани€ сложного соединени€ называетс€ тепловой эффект образовани€ 1 мол€ этого соединени€ из простых веществ, вз€тых в устойчивом состо€нии при заданных услови€х, то есть дл€ получени€ 1 мол€ Al2O3 необходимо 2 мол€ атомов Al, что составл€ет 54 г.
ѕо условию задачи:
при соединении 27 г алюмини€ выделитс€ 836,8 кƒж,
при соединении 54 г алюмини€ выделитс€ Q кƒж,
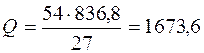 кƒж/моль,
кƒж/моль,
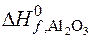 = Ц 1673,6 кƒж/моль.
= Ц 1673,6 кƒж/моль.
ѕример 3.3. –ассчитайте изменение внутренней энергии (Δ U) в химической реакции:
|
|
|
4NH3 (г) + 5O2 (г) = 4NO (г) + 6H2O (г).
– е ш е н и е
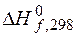 исходных веществ и продуктов реакции находим из табл. ѕ. 1.
исходных веществ и продуктов реакции находим из табл. ѕ. 1.
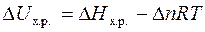 ,
,
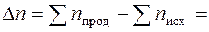 (4+6) Ц (4+5) = 1,
(4+6) Ц (4+5) = 1,
где n Ц число молей газообразных веществ.
ѕо закону √есса определ€ем энтальпию химической реакции
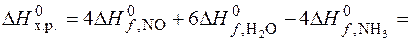 4Ј90,37 + 6Ј(-241,84) Ц Ц 4Ј(Ц46,19) = = Ц904,8 кƒж;
4Ј90,37 + 6Ј(-241,84) Ц Ц 4Ј(Ц46,19) = = Ц904,8 кƒж;
R = 8,31Ј10-3 ƒж/мольЈ ; “ = 298 ;
Δ U = Ц904,8 Ц 8,31Ј298Ј10-3 = Ц907,28 кƒж.
ѕример 3.4. –ассчитайте энтальпию образовани€ жидкого сероуглерода (CS2) по следующим данным:
S (ромб) + ќ2 (г) = SO2 (г) Δ Ќ 1 = -296,9 кƒж,
CS2 (ж) + 3ќ2 (г) = —ќ2 (г) + 2SO2 (г) Δ Ќ 2 = -1109,0 кƒж,
— (граф) + ќ2 (г) = —ќ2 (г) Δ Ќ 3 = -393,5 кƒж.
– е ш е н и е
—оставим термохимическое уравнение образовани€ CS2 (ж):
— (граф) + 2S (ромб) = —S2 (ж) + Δ Ќ х.
ƒл€ определени€ Δ Ќ х производим соответствующие алгебраические действи€ с термохимическими уравнени€ми, чтобы получить искомое, аналогичные действи€ производим и с Δ Ќ i:
 2 S(ромб) + ќ2 = SO2 (г) + Δ Ќ 1
2 S(ромб) + ќ2 = SO2 (г) + Δ Ќ 1
1 — (граф) + ќ2 = —ќ2 + Δ Ќ 3
- 1 CS2 (ж) + 3ќ2 = —ќ2 (г) + 2SO2 + Δ Ќ 2
 |





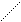 2S (pомб) + 3O2 + C (граф) Ц 3ќ2 Ц —S2 = 2SO2 + CO2 Ц CO2 Ц 2SO2 =
2S (pомб) + 3O2 + C (граф) Ц 3ќ2 Ц —S2 = 2SO2 + CO2 Ц CO2 Ц 2SO2 =
= 2 Δ Ќ 1 + Δ Ќ 3 Ц Δ Ќ 2
2S (ромб) + — (граф) = CS2 + 2 Δ Ќ 1 + Δ Ќ 3 Ц Δ Ќ 2
Δ Ќ x = Ц2 Δ Ќ 1 Ц Δ Ќ 3 + Δ Ќ 2 = Ц121,7 кƒж/моль.
ѕример 3.5. ¬ычислите 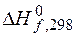 (кƒж),
(кƒж), 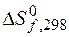 (ƒж/ ),
(ƒж/ ), 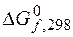 (кƒж) дл€ следующей реакции:
(кƒж) дл€ следующей реакции:
2—ќ (г) + 2Ќ2 (г) = —Ќ4 (г) + —ќ2 (г).
¬озможна ли реакци€ при – = 1 атм и “ = 298 ? ¬ычислите темпе-ратуру равновеси€ реакции и константы равновеси€ при 298 и 1000 .
– е ш е н и е
ѕо закону √есса определ€ем 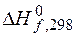 и
и 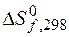 (термодина-мические параметры из табл.ѕ.1).
(термодина-мические параметры из табл.ѕ.1).
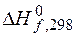 =
= 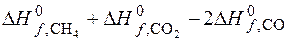 = -247,35 кƒж.
= -247,35 кƒж.
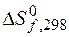 = -256,21 ƒж/ .
= -256,21 ƒж/ .
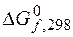 определ€ем по второму закону термодинамики:
определ€ем по второму закону термодинамики:
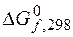 =
= 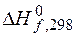 - 298Ј
- 298Ј 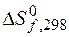 = Ц247,35 Ц 298Ј(Ц256,21) Ј10-3 = = Ц170,99 кƒж, т. е. реакци€ при 298 термодинамически веро€тна.
= Ц247,35 Ц 298Ј(Ц256,21) Ј10-3 = = Ц170,99 кƒж, т. е. реакци€ при 298 термодинамически веро€тна.
“емпература равновеси€ реакции Ц это “ равн, когда 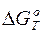 = 0.
= 0.
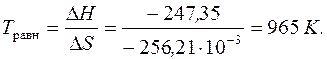
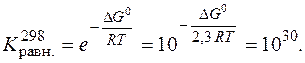 Ѕольшое значение
Ѕольшое значение 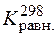 свидетельствует о том, что в стандартных услови€х идет практически только пр€ма€ реакци€.
свидетельствует о том, что в стандартных услови€х идет практически только пр€ма€ реакци€.
равн.при 1000 определ€ем по формуле
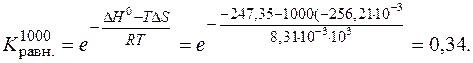
—ледовательно, при “ = 1000 в равновесной смеси преобладают исходные вещества.
ќтвет: 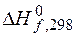 = Ц247,35 кƒж;
= Ц247,35 кƒж; 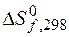 = Ц256 ƒж/ ;
= Ц256 ƒж/ ;
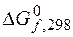 = Ц170,99 кƒж;
= Ц170,99 кƒж;
“ равн. = 965 ; 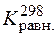 = 1030;
= 1030; 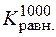 = 0,34.
= 0,34.
ѕример 3.6. акое количество продуктов необходимо употре-бить в день в определенном массовом соотношении, чтобы удовлетворить суточную энергетическую потребность организма?
ѕродукты: макаронные издели€, тел€тина филе, морковь, сыркова€ масса, бананы; массовое соотношение продуктов 3:1:2:1:4.
– е ш е н и е
ѕо таблице калорийности пищи (табл. ѕ.2) находим кало-рийность указанных продуктов: макаронные издели€ Ц 330 ккал/100 г, тел€тина филе Ц 158 ккал/100 г, морковь Ц 33 ккал/100 г, сыркова€ масса Ц 207 ккал/100 г, бананы Ц 91 ккал/100 г. “ак как массовое соотношение продуктов 3:1:2:1:4, то за единицу употреблени€ пищи принимаем следующие значени€: 300 г макаронных изделий, 100 г тел€тины, 200 г моркови, 100 г сырковой массы, 400 г бананов. ѕри употреблении такого количества пищи вы будете получать следующее количество энергии (Q) (ккал):
Q = 330,3 + 158,1 + 33,2 + 207,1 + 91,4 = 1758 ккал.
≈сли вы юноша, то в среднем потребл€йте Q ср. = 3300 ккал/сут, если девушка, то Q ср. = 2800 ккал/сут. Ќайдем коэффициент умножени€ продуктов (n)
n = Q ср./ Q.
Ќапример, вы юноша, тогда n = 3300/1758 = 1,87 раза, значит вам необходимо умножить единицу потреблени€ пищевых продуктов на величину 1,87, т. е. макаронных изделий необходимо употребить 300Ј1,87 = 561 г, тел€тины филе 100Ј1,87 = 187 г, моркови 200Ј1,87 = = 374 г, сырковой массы 100Ј1,87 = 187 г, бананов 400Ј1,87 = 748 г. Ёто то количество продуктов, которое вы должны употребить дл€ удовлетворени€ суточной энергетической потребности организма, при определенном массовом соотношении данных продуктов.
|
|
|
«адачи
3.1. ¬ычислите, сколько тепла выделитс€ при сгорании 100 л (н.у.) углеводорода. –еакци€ горени€ протекает по уравнению.
“ а б л и ц а 3.1
| ¬ариант | ”равнение |
| C2H6(г) + 3,5O2(г) = 2CO2(г) + 2H2O(ж) + 1559,88 кƒж | |
| C2H2(г) + 2,5O2(г) = 2CO2(г) + H2O(ж) + 1299,63 кƒж | |
| C2H4(г) + 3O2(г) = 2CO2(г) + 2H2O(ж) + 1410,97 кƒж | |
| C3H8(г) + 5O2(г) = 3CO2(г) + 4H2O(ж) + 2219,99 кƒж | |
| C3H6(г) + 4,5O2(г) = 3CO2(г) + 3H2O(ж) + 2017,64 кƒж | |
| C4H10(г) + 6,5O2(г) = 4CO2(г) + 5H2O(ж) + 3127,94 кƒж | |
| C4H8(г) + 6O2(г) = 4CO2(г) + 4H2O(ж) + 2718,51 кƒж | |
| C4H6(г) + 5,5O2(г) = 4CO2(г) + 3H2O(ж) + 2543,46 кƒж | |
| C5H12(г) + 8O2(г) = 5CO2(г) + 6H2O(ж) + 3536,19 кƒж | |
| C5H10(г) + 7,5O2(г) = 5CO2(г) + 5H2O(ж) + 3319,51 кƒж | |
| C3H4(г) + 4O2(г) = 3CO2(г) + 2H2O(ж) + 1944,31 кƒж | |
| C6H6(г) + 7,5O2(г) = 6CO2(г) + 3H2O(ж) + 2120,98 кƒж | |
| C6H12(г) + 9O2(г) = 6CO2(г) + 6H2O(ж) + 2772,47 кƒж | |
| C6H14(г) + 9,5O2(г) = 6CO2(г) + 7H2O(ж) + 3414,22 кƒж | |
| C6H8(г) + 8O2(г) = 6CO2(г) + 4H2O(ж) + 2331,97 кƒж |
ѕри соединении определенного количества вещества с кислородом выделилась теплота. ќпределите энтальпию образовани€ оксидов.
“ а б л и ц а 3.2
| ¬ариант | ¬ещество | ћасса, г | ¬ыделенна€ теплота, кƒж |
| 1Т | Al | 836,8 | |
| 2Т | B | 27,5 | 1569,0 |
| 3Т | P | 6192,3 | |
| 4Т | Fe | 4100,3 | |
| 5Т | Si | 2,8 | 87,2 |
| 6Т | Li | 1190,8 | |
| 7Т | Ca | 2538,9 | |
| 8Т | Fe | 539,7 | |
| 9Т | S | 1485,3 | |
| 10Т | Na | 416,3 | |
| 11Т | K | 780,0 | |
| 12Т | Cr | 285,3 | |
| 13Т | Zn | 40,2 | |
| 14Т | Ca | 14,0 | |
| 15Т | Mg | 300,5 |
3.2. Ќе провод€ расчета определите, дл€ каких из нижеприве-денных реакций разница в значени€х D H и D U будет равна нулю.
“ а б л и ц а 3.3
| ¬ариант | ”равнени€ реакций | ¬ариант | ”равнени€ реакций |
| ј. — (т) + 1/2 ќ2 (г) Ѓ —ќ (г) Ѕ. — (т) + ќ2 (г) Ѓ —ќ2 (г) ¬. 2— (т) + 3Ќ2 (г) Ѓ —2Ќ6 (г) | 1Т | ј. 1/2N2 (г) + 3/2 H2(г) Ѓ NH3(г) Ѕ. N2 (г) + 2H2 (г) Ѓ N2H4 (г) ¬. N2(г)+ 2Ќ2(ж) Ѓ N2H2 (г) | |
| ј. S (тв) + O2 (г) Ѓ SO2 (г) Ѕ. S (тв) + 3/2O2 (г) Ѓ SO3 (г) ¬. S (тв) + H2 (г) Ѓ H2S (г) | 2Т | ј. 1/2N2 (г) + 1/2O2 (г) Ѓ NO (г) Ѕ. 1/2N2 (г) + O2 (г) Ѓ NO2 (г) ¬. N2 + 5/2O2 (г) Ѓ N2O5 (г) | |
| ј. H2 (г) + O2 (г) Ѓ H2O2 (г) Ѕ. H2 (г) + 1/2O2 (ж) Ѓ H2O (г) ¬. H2 (г) + 1/2O2 (г) Ѓ H2O (ж) | 3Т | ј. Al (тв) + 1/2Cl2 (г) Ѓ AlCl (г) Ѕ. Al (тв) + 3/2Cl2 (г) Ѓ AlCl3 (г) ¬. Al(тв) + 3/2Cl2(ж) Ѓ AlCl3(тв) |
Ќе провод€ расчета определите, дл€ каких из нижеприведенных реакций разница в значени€х Qv и Qp будет наибольшей.
“ а б л и ц а 3.4
| ¬ариант | ”равнени€ реакций | ¬ариант | ”равнени€ реакций |
| ј. Cl2 (г) + 1/2O2 (г) Ѓ Cl2O (г) Ѕ. Cl2 (г) + 5/2O2 (г) Ѓ Cl2O5 (г) ¬. Cl2 (г) + 3/2O2 (г) Ѓ Cl2O3 (г) | 4Т | ј. C (тв) + 2H2 (г) Ѓ CH4 (г) Ѕ. 2C (тв) + 3H2 (г) Ѓ C2H6 (г) ¬. 6C (тв) + 3H2 (г) Ѓ C6H6 (г) |
–ассчитайте изменение внутренней энергии (кƒж) в химической реакции.
“ а б л и ц а 3.5
| ¬ариант | ”равнени€ реакций | ¬ариант | ”равнени€ реакций |
| 2—l2 + 2Ќ2ќ(г) = 4Ќ—l (г) + ќ2 | 5Т | 2CO(г) + 2H2(г) = CH4(г) + CO2(г) | |
| CO2 (г) + C (к) = 2CO (к) | 6Т | 2SO2 (г) + O2 (г) = 2SO3 (г) | |
| 2CH4(г) = C2H4(г) + 2H2(г) | 7Т | COCl2(г) = CO(г) + Cl2(г) |
Ќе производ€ вычислений установите знак 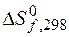 следующих процессов.
следующих процессов.
“ а б л и ц а 3.6
|
|
|
| ¬ариант | ”равнени€ реакций | ¬ариант | ”равнени€ реакций |
| Cl (тв) Ѓ Cl (водн) CaCO3 (тв) Ѓ CaO(тв) + CO2(г) | 8Т | N2 (г) + 3 H2 (г) Ѓ 2 NH3 (г) Ag+(водн) + Cl- (водн) Ѓ Ѓ AgCl (тв) | |
| CuO (тв) Ѓ Cu(тв) + 1/2 O2(г) Cl2 (г) Ѓ 2 Cl (г) | 9Т | CO2(к) = CO2(г) 2NO(г) + O2(г) = 2NO2(г) | |
| 2H2S(г) + 3O2 = 2H2O(ж) + + 2SO2(г) | 10Т | MgO(к) + H2(г) = Mg(к) + + H2O(ж) | |
| C(граф) + CO2 = 2CO(г) | 11Т | CH3COOH(водн) = = CH3COO-(водн) + H+(водн) | |
| ќкончание табл. 3.6 | |||
| 4HCl(г) + O2(г) = 2Cl2(г) + +2H2O(г) | 12Т | NH4NO3(к) = N2O(г) + 2H2O(г) | |
| 2CO(г) + 2H2(г) = CH4(г) + +CO2(г) | 13Т | COCl2 (г) = CO (г) + Cl2 (г) | |
| 2SO2 (г) + O2 (г) = 2SO3 (г) | 14Т | CH4(г) + 2O2(г) = CO2(г) + +2H2O(г) | |
| 2CH4 (г) = C2H2 (г) + 3H2 (г) 2CH4 (г) = C2H4 (г) + 2H2 (г) | 15Т | 2CO (г) + O2 (г) = 2CO2 (г) CO (г) + H2O (г) = CO2 (г) + +H2 (г) |
3.3. «на€ теплоты сгорани€ графита и алмаза, вычислите неподдающуюс€ экспериментальному определению теплоту превращени€ графита в алмаз:
— (графит) + ќ2 = —ќ2 + 393,51 кƒж,
— (алмаз) + ќ2 = —ќ2 + 395,40 кƒж.
3.4. ќпределите энтальпию гидратации соды по реакции
Na2CO3 + 10H2O = Na2CO3 × 10H2O,
если при растворении безводной соды
Na2CO3 + aq = Na2CO3 × aq D H 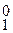 = Ц 25,12 кƒж
= Ц 25,12 кƒж
и при растворении кристаллогидрата
Na2CO3 × 10H2O + aq = Na2CO3 × aq + 10H2O D H 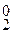 = 66,99 кƒж.
= 66,99 кƒж.
3.5. ƒл€ реакции 2O3 (г) = 3O2 (г) D H 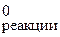 = Ц 288,90 кƒж.
= Ц 288,90 кƒж.
»спользу€ D H 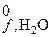 , вычислите изменение энтальпии в реакции
, вычислите изменение энтальпии в реакции






