ќкислительно-восстановительными называютс€ реакции, в которых происходит изменение степеней окислени€ атомов элементов, вход€щих в состав реагирующих соединений, при этом электроны от одних атомов, молекул или ионов переход€т к другим. ѕри этом выдел€ют два сопр€женных процесса: окисление и восстановление.
ќкисление Ц реакци€, отвечающа€ потере (отдаче) электронов атомами элемента. Ќапример, в реакции
| 2H2SЦ2 + 3O20 = 2S+4O2Ц2 + 2H2O | (1) |
процесс окислени€
SЦ2 Ц 6 еЦ = S+4.
¬осстановление Ц реакци€, сопровождающа€с€ присоединением (вз€тием) электронов атомами этого элемента; в указанной выше реакции процесс восстановлени€
O20 + 2 еЦ = 2OЦ2.
Ёлементы, вступающие в процесс окислени€ и восстановлени€, называютс€ окислител€ми и восстановител€ми. ќкислитель Ц вещество (молекула, атом или ион), котороеприсоедин€ет электроны (восстанавливаетс€) и понижает свою степень окислени€. ¬осстановитель Цвещество (молекула, атом или ион), которое отдает электроны (окисл€етс€) и повышает свою степень окислени€. Ќапример, в реакции (1)
H2S Ц восстановитель, ќ2 Ц окислитель.
¬ окислительно-восстановительных реакци€х число электронов, отдаваемых восстановителем, равно числу электронов, присоедин€емых окислителем.
Ёлементы, наход€щиес€ в высшей степени окислени€, могут только восстанавливатьс€, так как их атомы способны только принимать электроны. Ќапротив, элементы, наход€щиес€ в низшей степени окислени€, могут только окисл€тьс€, поскольку их атомы способны только отдавать электроны. ¬ещества, содержащие элементы в промежуточных степен€х окислени€, обладают окислительно-восстановительной двойственностью. “акие вещества способны и принимать, и отдавать электроны в зависимости от партнера, с которым они взаимодействуют, и от условий реакции.
¬ уравнени€х окислительно-восстановительных реакций коэффициенты могут быть установлены с помощью нескольких методов. –ассмотрим один из них.
Ёлектронный баланс Ц метод нахождени€ коэффициентов в уравнени€х окислительно-восстановительных реакций, в котором рассматриваетс€ обмен электронами между атомами элементов, измен€ющих свою степень окислени€.
”равнение составл€етс€ в несколько стадий.
1. «аписывают схему реакции:
KMnO4 + HCl → KCl + MnCl2 + Cl2 + H2O.
2. ќпредел€ют степени окислени€ всех элементов:
K+1Mn+7O4Ц2+ H+1ClЦ1→ K+1ClЦ1+ Mn+2Cl2Ц1+ Cl20+ H2+1OЦ2.
3. ¬ыдел€ют элементы, измен€ющие степени окислени€:
K+1 Mn+7 O4Ц2+ H+1 ClЦ1 → K+1ClЦ1+ Mn+2 Cl2Ц1+ Cl20 + H2+1OЦ2.
4. ќпредел€ют число электронов, приобретенных окислителем и отдаваемых восстановителем:
Mn+7+ 5 еЦ → Mn+2,
2ClЦ1Ц 2 еЦ → Cl20.
5. ”равнивают число приобретенных и отдаваемых электронов, устанавлива€ тем самым коэффициенты дл€ соединений, в которых присутствуют элементы, измен€ющие степень окислени€:
| Mn+7+ 5 еЦ → Mn+2 | Ј2 | процесс восстановлени€, Mn+7Ц окислитель |
| 2ClЦ1Ц 2 еЦ → Cl20 | Ј5 | процесс окислени€, ClЦ1Ц восстановитель |
2Mn+7+ 10ClЦ1→ 2Mn+2+ 5Cl20.
|
|
|
6. ѕодбирают коэффициенты дл€ всех остальных участников реакции в следующей последовательности: металлы, неметаллы, кислород, водород:
2KMnO4 + 16HCl = 2KCl + 2MnCl2 + 5Cl2 + 8H2O.
—уществуют несколько типов окислительно-восстанови-тельных реакций (ќ¬–).
1. ћежмолекул€рные ќ¬–Цреакции, в которыхокислитель и восстановитель наход€тс€ в разных веществах; обмен электронами в этих реакци€х происходит между различными атомами или молекулами.
S0+ O20= S+4O2Ц2,
| O20+ 2 еЦ = 2OЦ2Ц процесс восстановлени€, O20Ц окислитель, |
| S0Ц 4 еЦ = S+4Ц процесс окислени€, S0Ц восстановитель. |
Cu+2O + C+2O = Cu0+ C+4O2,
| Cu+2+ 2 еЦ = Cu0Ц процесс восстановлени€, Cu+2Ц окислитель, |
| C+2Ц 2 еЦ = —+4Ц процесс окислени€, C+2Ц восстановитель. |
Mn+4O2 + 2KIЦ1+ 2H2SO4 = I20+ K2SO4 + Mn+2SO4 + 2H2O,
| Mn+4+ 2 еЦ = Mn+2Ц процесс восстановлени€, Mn+4Ц окислитель, |
| 2IЦ1Ц 2 еЦ = I20Ц процесс окислени€, IЦ1Ц восстановитель. |
2. ¬нутримолекул€рные ќ¬– Цреакции, в которыхокислитель и восстановитель наход€тс€ в одной и той жемолекуле. ¬нутримолекул€рные реакции протекают, как правило, при термическом разложениивеществ, содержащих окислитель и восстановитель.
2KCl+5O3Ц2= 2KClЦ1+ 3O20,
| Cl+5+ 6 еЦ = ClЦ1Ц процесс восстановлени€, Cl+5Ц окислитель, |
| 2ќЦ2Ц 4 еЦ = ќ20Ц процесс окислени€, ќЦ2Ц восстановитель. |
NЦ3H4N+5O3 = N2+1O + 2H2O,
| N+5+ 6 еЦ = N+1Ц процесс восстановлени€, N+5Ц окислитель, |
| NЦ3Ц 4 еЦ = N+1Ц процесс окислени€, NЦ3Ц восстановитель. |
2Pb(N+5O3Ц2)2 = 2PbO + 4N+4O2 + O20,
| N+5+ 1 еЦ = N+4Ц процесс восстановлени€, N+5Ц окислитель, |
| 2OЦ2Ц 4 еЦ = O20Ц процесс окислени€, OЦ2Ц восстановитель. |
3. –еакции диспропорционировани€ (дисмутации) Цќ¬–, в которыходин элемент одновременно повышает и понижает степень окислени€.
Cl20+ 2KOH = KCl+1O + KClЦ1+ H2O,
| Cl20+ 2 еЦ = 2Cl+1Ц процесс восстановлени€, Cl20Ц окислитель, |
| Cl20Ц 2 еЦ = 2ClЦ1Ц процесс окислени€, Cl20Ц восстановитель. |
3HN+3O2 = HN+5O3 + 2N+2O + H2O,
| N+3+ 1 еЦ = N+2Ц процесс восстановлени€, N+3Ц окислитель, |
| N+3Ц 2 еЦ = N+5Ц процесс окислени€, N+3Ц восстановитель. |
4. –еакции конпропорционировани€ (конмутации) Цќ¬– между двум€ веществами, в которых атомы одного и того же элемента имеют разные степени окислени€.
2H2SЦ2+ H2S+4O3 = 3S0+ 3H2O,
| S+4+ 4 еЦ = S0Ц процесс восстановлени€, S+4Ц окислитель, |
| SЦ2Ц 2 еЦ = S0Ц процесс окислени€, SЦ2Ц восстановитель. |
“ипичные окислители
1. Ќеметаллы: F2, Cl2, Br2, I2, O2, водород в степени окислени€ +1.
2. »оны металлов в высшей степени окислени€, Fe+3, Cu+2, Hg+2.
3. ислородсодержащие кислоты: H2SO4, HNO3, HMnO4 и их соли: Na2SO4, KMnO4, K2CrO7.
4. ислородсодержащие кислоты галогенов: HClO, HClO3, HBrO3.
“ипичные восстановители
1. јктивные металлы: щелочные, щелочноземельные металлы, Zn, Al, Fe.
2. Ѕескислородные кислоты: HCl, HBr, HJ, H2S.
3. √идриды щелочных и щелочноземельных металлов: NaH, CaH2.
4. ћеталлы в низшей степени окислени€: Sn+2, Fe+2, Cu+.
–€д веществ, имеющих элементы, наход€щиес€ в промежуточных степен€х окислени€, способны про€вл€ть как окислительные, так и восстановительные свойства (окислительно-восстановительна€ двойственность). Ќапример, кислород в пероксиде водорода H2O2 в присутствии восстановителей может понижать степень окислени€ от Ц1 до Ц2 (окислитель); а при взаимодействии с окислител€ми Ц повышать степень окислени€ от Ц1 до 0 (восстановитель).
|
|
|
5H2O2Ц1+ J20= 2HJ+5O3 + 4H2OЦ2,
H2O2 Ц окислитель;
3H2O2Ц1+ 2KMnO4 = 2MnO2 + 2KOH + 3O20+ 2H2O,
H2O2 Ц восстановитель.
ќсновные пон€ти€ электрохимических процессов
Ёлектрохимическими процессами называют процессы взаимного превращени€ химической и электрической форм энергии. Ёлектрохимические процессы можно разделить на две основные группы: процессы превращени€ химической энергии в электрическую (в гальванических элементах); процессы превращени€ электрической энергии в химическую (электролиз).
Ёлектрохимические реакции, протекающие в гальванических элементах и при электролизе, проход€т на границе раздела веществ, имеющих ионную (например, растворы электролитов) и электронную проводимость (например, металлы).
–ассмотрим процессы, протекающие на границе раздела металл Ц раствор соли металла. ѕри погружении металла в раствор начинаетс€ взаимодействие металла с компонентами раствора:
M ⇄ M n + + nеЦ.
ѕр€ма€ реакци€ Ц окисление металла и диффузи€ его гидратированных ионов в раствор, обратна€ реакци€ Ц восстановление ионов металла из раствора на поверхности металлического кристалла.
–ассмотрим случай, когда скорость пр€мой реакции больше скорости обратной. ћеталл, окисл€€сь, оставл€ет в кристалле электроны. ѕоверхность металла из-за избытка электронов зар€жаетс€ отрицательно. ѕоложительно зар€женные ионы из раствора (катионы металла, диполи воды) прит€гиваютс€ к отрицательной поверхности металла, на границе возникает двойной электрический слой (рис. 5.3.1, а).
¬ случае, если скорость обратной реакции больше скорости пр€мой, катионы соли, восстанавлива€сь на поверхности металлической поверхности, Ђобедн€ютї ее электронами и металл зар€жаетс€ положительно. ќтрицательно зар€женные ионы из раствора (анионы соли металла, диполи воды) прит€гиваютс€ к положительно зар€женной поверхности металла, на границе возникает двойной электрический слой (рис. 5.3.1, б).

“аким образом, между металлом и раствором в услови€х равновеси€ возникает разность потенциалов, котора€ называетс€ равновесным электродным потенциалом. јбсолютные значени€ электродных потенциалов экспериментально определить невозможно, их можно только сравнить.


¬ качестве электрода сравнени€ используют водородный электрод. ѕотенциал стандартного водородного электрода при температуре 298 K условно принимают равным нулю.
¬одородный электрод состоит из платинированной платины, полученной нанесением на поверхность платины сло€ высокодисперсной платины, контактирующей с газообразным водородом, наход€щимс€ под давлением 100 кѕа, и раствором, в котором концентраци€ ионов Ќ+ равна единице. ѕри контакте платины с молекул€рным водородом происходит адсорбци€ водорода на платине. јдсорбированный (поглощенный поверхностью) водород, взаимодейству€ с молекулами воды, переходит в раствор в виде ионов, оставл€€ в платине электроны. Ќар€ду с переходом ионов в раствор идет обратный процесс восстановлени€ ионов Ќ+ с образованием молекул водорода. –авновесие на водородном электроде можно записать в виде
2Ќ+ + 2 еЦ ⇄ Ќ2.
ƒл€ определени€ потенциалов электродов по водородной шкале собирают гальванический элемент, состо€щий из водородного электрода и исследуемого металла (рис. 5.3.2).
Ёлектродный потенциал металла, измеренный по отношению к водородному электроду при стандартных услови€х (т. е. при концентрации ионов металлов в растворе 1 моль/л и температуре 298 ), называют стандартным электродным потенциалом металла (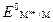 , ¬). «начени€ стандартных электродных потенциалов металлов Ц справочна€ величина.
, ¬). «начени€ стандартных электродных потенциалов металлов Ц справочна€ величина.
≈сли услови€ отличаютс€ от стандартных, то дл€ расчета электродных потенциалов используют уравнение Ќернста:
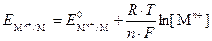 , ,
| (1) |
где 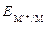 Ц электродный потенциал металла, ¬;
Ц электродный потенциал металла, ¬; 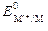 Ц его стандартный электродный потенциал, ¬; R Ц универсальна€ газова€ посто€нна€ (8,314 ƒж /(мольЈ )); T Ц температура, ; n Ц число электронов, принимающих участие в процессе; F Ц число ‘араде€ (96485 л/моль); [M n +] Ц концентраци€ ионов в растворе, моль/л.
Ц его стандартный электродный потенциал, ¬; R Ц универсальна€ газова€ посто€нна€ (8,314 ƒж /(мольЈ )); T Ц температура, ; n Ц число электронов, принимающих участие в процессе; F Ц число ‘араде€ (96485 л/моль); [M n +] Ц концентраци€ ионов в растворе, моль/л.
|
|
|
ѕереход€ от натурального логарифма к дес€тичному и подставл€€ в уравнение (9.3.1) соответствующие значени€ R, F и “ = 298 , получаем
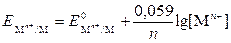 . .
| (2) |
—огласно уравнению Ќернста потенциал металлического электрода зависит от концентрации ионов металлов в растворе, от температуры и от природы металла.
ѕотенциалы газовых электродов. √азовые электроды состо€т из металлического проводника, контактирующего одновременно с газом и раствором, содержащим ионы этого газа. ћеталл €вл€етс€ только электронным проводником и не принимает участи€ в окислительно-восстановительных реакци€х (электрохимически инертен), например, платина и платиновые металлы, графит, стеклоуглерод. “ак как в равновесных электродных реакци€х участвуют газообразные компоненты, то электродные потенциалы этих электродов завис€т от парциальных давлений газов.
ѕотенциалы окислительно-восстановительных (редокси-) электродов. окислительно-восстановительным (редокси-) электродам относ€т только те электроды, в реакци€х которых не принимают непосредственного участи€ металлы и газы. “акие электроды состо€т из металлического проводника, контактирующего с раствором, содержащим окислители и восстановители. металлу в редокси-электродах предъ€вл€ютс€ те же требовани€, что и к металлическому проводнику в газовых электродах.
¬ общем виде равновесие на электроде дл€ простых систем записываетс€ уравнением
Ox + n еЦ Ѓ Red,
Red Ц n еЦ Ѓ Ox,
где Ox Ц окисленна€ форма вещества; Red Ц восстановленна€ форма вещества.
”равнение Ќернста дл€ расчета потенциала редокси-электрода имеет вид
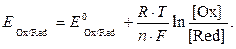
| (3) |
«начени€ стандартных потенциалов редокси-электродов можно найти в справочниках. ѕотенциал окислительно-восстановительных электродов служит мерой окислительной и восстановительной способности систем. ќкислительна€ способность систем возрастает со сдвигом редокси-потенциала в сторону положительных значений. ¬осстановительна€ способность систем растет со сдвигом потенциала в сторону отрицательных значений.
–€д напр€жений металлов
¬се элементы периодической системы можно расположить в определенной последовательности согласно увеличению значени€ электродного потенциала, называемой р€дом напр€жений металлов.
„тобы рационально использовать р€д напр€жений (–Ќ) необходимо помнить следующее:
1. „ем левее расположен металл в р€ду напр€жений, тем он химически активнее, обладает большей восстановительной способностью, легче окисл€етс€ и труднее восстанавливаетс€ из его ионов.
2. „ем правее расположен металл в р€ду напр€жений, тем он химически менее активен, труднее окисл€етс€ и легче восстанавливаетс€ из его ионов.
3. ¬се металлы с отрицательной величиной электродного потенциала, расположенные левее водорода, окисл€ютс€ ионами гидроксони€ и выдел€ют водород из разбавленных растворов кислот, анионы которых не про€вл€ют окислительных свойств. Ќапример,
Mg + 2H2O = Mg(OH)2 + H2, 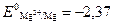 ¬;
¬;
Cu + H2O ≠, 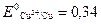 ¬.
¬.
4. ћеталлы, сто€щие в р€ду напр€жений левее, вытесн€ют металлы, сто€щие правее, из растворов их солей. Ќапример,
MgSO4 + Cu ≠,
Mg + CuSO4 = MgSO4 + Cu.
5. ћеталлы, сто€щие в р€ду напр€жений, условно дел€т на три группы: активные металлы (от Li до Al); металлы средней активности (от Ti до водорода); малоактивные металлы (после водорода).
√альванический элемент
≈сли мы опустим цинковую пластинку в раствор сульфата меди, то произойдет реакци€
Zn + CuSO4 = Zn SO4+ Cu.
¬ результате реакции выделитс€ теплота (Q). “о есть химическа€ энерги€ перейдет в теплоту. ћожно перевести химическую энергию в электрическую.
|
|
|
√альванический элемент Ц устройство, в котором химическа€ энерги€ окислительно-восстановительной реакции превращаетс€ в электрическую энергию.
ќсновным отличием электрохимических реакций, протекающих в гальваническом элементе, от окислительно-восстано-вительных реакций €вл€етс€ пространственное разделение процессов окислени€ и восстановлени€.
Ќаиболее распространенным €вл€етс€ гальванический элемент, состо€щий из двух св€занных между собой электродов, представл€ющих собой металлические пластины, погруженные в раствор электролита (растворы или расплавы солей с одноименным ионом).
–ассмотрим работу гальванического элемента ƒаниэл€Цякоби (рис. 955.1), состо€щего из двух электродов Ц цинковой пластины, погруженной в раствор сульфата цинка, и медной пластины, погруженной в раствор сульфата меди. ќба раствора соприкасаютс€ друг с другом, но дл€ предупреждени€ смешивани€ они разделены перегородкой, изготовленной из пористого материала.
ѕри разомкнутой цепи в этом гальваническом элементе устанавливаетс€ равновесие между цинковым электродом и раствором сульфата цинка, а также между медным электродом и раствором сульфата меди. ѕри замыкании внешней цепи электроны перемещаютс€ от электрода с более низким потенциалом (цинкового) к электроду с более высоким потенциалом (медному).
Ќа цинковом электроде протекает реакци€ окислени€, и ионы переход€т в раствор. ¬ысвобождающиес€ при этом электроны движутс€ по внешней цепи к медному электроду. ¬с€ совокупность этих процессов схематически изображаетс€ уравнением первой полуреакции
Zn Ц 2 e Ц = Zn2+.
Ќа медном электроде протекает восстановление ионов меди: электроны, переместившиес€ к нему от цинкового электрода, соедин€ютс€ с наход€щимис€ в растворе ионами меди; образуютс€ атомы меди, выдел€ющиес€ в элементной форме на пластине металла. —оответствующа€ втора€ полуреакци€ Ц
Cu2++ 2 e Ц = Cu0.
—уммарное уравнение реакции, протекающей в элементе, получитс€ при сложении уравнений обеих полуреакций
Cu2+ + 2 e Ц + Zn0Ц 2 e Ц = Zn2+ + Cu0.


Ќаправление движени€ ионов в растворе обусловлено протекающими у электродов электрохимическими процессами. ак было упом€нуто выше, у цинкового электрода катионы (положительные зар€женные ионы) выход€т в раствор, создава€ в нем избыточный положительный зар€д, а у медного электрода раствор, наоборот, все врем€ обедн€етс€ катионами и зар€жаетс€ отрицательно. ¬ результате этого создаетс€ электрическое поле, в котором катионы (Zn2+ и Cu2+) движутс€ от цинкового электрода к медному, а анионы Ц SO42Ц Ц в обратном направлении. ¬ итоге жидкость у обоих электродов остаетс€ электронейтральной.
ѕричиной возникновени€ и протекани€ электрического тока в гальваническом элементе €вл€етс€ разность электродных потенциалов Ц электродвижуща€ сила (Ёƒ—). Ёƒ— любого гальванического элемента равна разности потенциалов двух его электродов (катода и анода):
| Ёƒ— = ≈ к Ц ≈ а > 0, | (5) |
где ≈ к Ц электродный потенциал катода; ≈ а Ц электродный потенциал анода. Ёƒ— любого работающего гальванического элемента Ц величина положительна€.
јнод Ц электрод, на котором происходит процесс отдачи электронов (окисление); в гальваническом элементе анод зар€жен отрицательно. атод Ц электрод, на котором происходит процесс присоединени€ электронов (восстановление); в гальваническом элементе катод зар€жен положительно.
ѕотенциал катода больше потенциала анода, соответственно, в медно-цинковом элементе цинковый электрод €вл€етс€ анодом, а медный Ц катодом.
ƒл€ элемента ƒаниэл€Цякоби при стандартных услови€х и при [Zn2+] = [Cu2+] = 1 моль/л
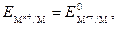
поэтому Ёƒ— = 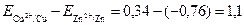 ¬.
¬.
—хема цепи гальванического элемента записываетс€ в виде
(Ц) Zn | Zn2+|| Cu2+| Cu (+)
или
(Ц) Zn | ZnSO4|| CuSO4 | Cu (+).
—лева записываетс€ анод, а справа Ц катод. ќдна вертикальна€ черта изображает фазовый раздел между металлом и раствором электролита. ƒвойна€ вертикальна€ лини€ отдел€ет анодное пространство от катодного. ¬ круглых скобках знаками Ђплюсї и Ђминусї обозначают полюсы электродов.
онцентрационный гальванический элемент представл€ет собой металлический гальванический элемент, составленный из двух одинаковых по природе электродов, погруженных в растворы своих солей с различными концентраци€ми. Ќапример, серебр€ный гальванический элемент
(Ц) Ag | AgNO3 (0,001 M) || AgNO3 (0,1M) | Ag (+).
«десь левый электрод с меньшей концентрацией €вл€етс€ анодом, а правый Ц с большей концентрацией Ц катодом.
¬ некоторых случа€х металл электрода не претерпевает изменений в ходе электродного процесса, а участвует только в передаче электронов от восстановленной формы вещества к его окисленной форме. “ак, в гальваническом элементе
|
|
|
(Ц) Pt | Fe+2, Fe+3 || MnO4Ц, Mn+2 , H+ |Pt (+)
роль инертных электродов играет платина. Ќа платиновом аноде окисл€етс€ железо (II):
Fe+2 = Fe+3 + e Ц,
а на платиновом катоде восстанавливаетс€ марганец (VII):
MnO4Ц+ 8H++ 5 e Ц = Mn+2+ 4H2O.
”множив первое из этих уравнений на п€ть и сложив со вторым, получаем суммарное уравнение протекающей реакции:
5Fe+2+ MnO4Ц+ 8H+= 5Fe+3+ Mn+2+ 4H2O.
—огласно уравнению 9.3.3. можно рассчитать электродные потенциалы и, соответственно, Ёƒ— полученного гальванического элемента.
Ёлектролиз
Ёлектролиз Ц окислительно-восстановительный процесс, который протекает на электродах при прохождении посто€нного электрического тока через растворы или расплавы электролитов. —ущность электролиза заключаетс€ в том, что при пропускании тока через раствор электролита (или расплавленный электролит) катионы перемещаютс€ к отрицательному электроду (катоду), а анионы Ц к положительному электроду (аноду). ƒостигнув электродов, ионы разр€жаютс€, в результате чего у электродов выдел€ютс€ составные части растворенного электролита или водород и кислород из воды. ѕри электролизе протекают два параллельных процесса: на катоде (зар€жен отрицательно) процесс восстановлени€; на аноде (зар€жен положительно) Ц процесс окислени€. “аким образом, зар€ды электродов при электролизе противоположны тем, которые имеют место при работе гальванического элемента.
Ќа характер и течение электродных процессов при электролизе большое вли€ние оказывают состав электролита, растворитель, материал электродов и режим электролиза (напр€жение, плотность тока, температура и др.). ѕрежде всего, надо различать электролиз расплавленных электролитов и растворов.
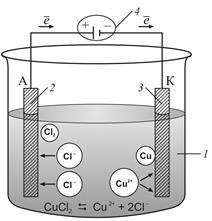
|
| –ис. 5.6.1. —хема процесса электролиза расплава CuCl2: 1 Ц расплав соли CuCl2; 2 Ц анод; 3 Ц катод; 4 Ц источник посто€нного тока |
Ёлектролиз расплавов солей. –ассмотрим в качестве примера электролиз расплава хлорида меди (рис. 5.6.1). ѕри высоких температурах расплав соли диссоциирует на ионы. ѕри подключении электродов к источнику посто€нного тока ионы под действием электрического пол€ начинают упор€доченное движение: положительные ионы меди движутс€ к катоду, а отрицательно зар€женные ионы хлора Ц к аноду.
ƒостигнув катода, ионы меди нейтрализуютс€ избыточными электронами катода и превращаютс€ в нейтральные атомы, оседающие на катоде:
Cu+2 + 2 e Ц → Cu0.
»оны хлора, достигнув анода, отдают электроны и образуют молекулы хлора Cl2. ’лор выдел€етс€ на аноде в виде пузырьков:
2Cl ЦЦ 2 e Ц → 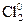 .
.
—уммарное уравнение окислительно-восстановительной реакции, происход€щей при электролизе расплава CuCl2:
Cu+2 + 2ClЦ → Cu0 + 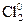 .
.
Ёлектролиз водных растворов солей. ¬ водных растворах, кроме ионов самого электролита, наход€тс€ также молекулы воды, способные восстанавливатьс€ на катоде и окисл€тьс€ на аноде.
ѕроцессы на катоде. ¬озможность протекани€ восстановлени€ ионов металла или молекул воды определ€етс€ значением электродного потенциала металла, а также характером среды (рЌ). ¬ общем случае (без вли€ни€ характера среды) на катоде могут протекать следующие процессы (табл. 1):
“аблица 1
—хема процессов, протекающих на катоде
| Li, Rb, K, Cs, Ba, Sr, Ca, Na, Mg, Be, Al | |
| ¬осстановление молекул воды 2H2O + 2 e Ц → H2 + 2OHЦ | |
| Ti, Mn, Cr, Zn, Fe, Cd, Co, Ni, Sn, Pb,(H) | |
| ¬осстановление молекул воды и катиона металла 2H2O + 2 e Ц → H2 + 2OHЦ; M n ++ ne Ц → M0 | |
| Sb, Bi, Cu, Hg, Ag, Pd, Pt, Au | |
| ¬осстановление катиона металла M n + + ne Ц → M0 |
1) если электролизу подвергаетс€ соль активного металла, то на катоде восстанавливаютс€ молекулы воды. ¬ результате у катода выдел€етс€ водород;
2) если электролизу подвергаетс€ соль среднеактивного металла, то происходит одновременное восстановление и катионов металла, и молекул воды;
3) если электролизу подвергаетс€ соль малоактивного металла, то на катоде восстанавливаютс€ только катионы металла.
ѕроцессы на аноде. ѕри рассмотрении анодных процессов следует учитывать тот факт, что материал анода в ходе электролиза может окисл€тьс€. ѕоэтому различают электролиз с инертным анодом и электролиз с активным анодом.
»нертным называетс€ анод, материал которого в процессе электролиза химически не измен€етс€. ƒл€ изготовлени€ инертных анодов обычно примен€ют графит, уголь, платину. Ќа инертном аноде при электролизе водных растворов могут протекать процессы (табл. 2):
“аблица 2
—хема процессов, протекающих на аноде
| S2Ц, IЦ, BrЦ, ClЦ | |
| ќкисление кислотного остатка X n ЦЦ ne Ц→ X0 | |
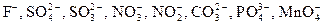
| |
| ќкисление молекул воды 2H2O Ц 4 e Ц → O2 + 4H+ |
Ц если электролизу подвергаетс€ соль бескислородной кислоты, то на аноде окисл€етс€ анион кислотного остатка. »сключением €вл€етс€ фтор-анион, имеющий высокий окислительный потенциал;
Ц если электролизу подвергаетс€ соль кислородсодержащей кислоты или сама кислота, то на аноде окисл€ютс€ молекулы воды. ¬ результате у анода выдел€етс€ кислород.
јктивным называетс€ анод, материал которого (металл) входит в состав электролизуемой соли. ѕри этом материал анода окисл€етс€ и металл переходит в раствор в виде ионов, т. е. окисл€етс€. јктивные аноды изготавливают из Cu, Ag, Zn, Cd, Ni, Fe и т. д. ƒл€ примера приведем электролиз нитрата серебра (AgNO3) с нерастворимым и растворимым анодами (Ag):
| »нертный анод: | јктивный анод (Ag): |
| (Ц): Ag1+ +1 e Ц → Ag0 ј (+): 2H2O Ц 4 e Ц → O2 + 4H + | (Ц): Ag1+ +1 e Ц → Ag0 ј (+): Ag0 Ц 1 e Ц → Ag1+ |
ѕроцессы электролиза характеризуютс€ законами ‘араде€, определ€ющими зависимость между количеством прошедшего электричества и количеством вещества, испытывающего химические превращени€ на электроде.
1-й закон ‘араде€. оличество вещества, выдел€емое на электроде, пр€мо пропорционально количеству пропущенного электричества.
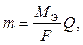
| (6) |
где m Ц масса вещества, испытывающего электрохимическое превращение; M Ё Ц эквивалентна€ мол€рна€ масса вещества; F Ц посто€нна€ ‘араде€, 96500 л; Q Ц количество электричества.
“ак как Q=I×t,где I Ц сила токај, t Ц врем€,с, формулу 9.6.1 можно переписать в следующем виде
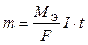 . .
| (7) |
ќбычно количество вещества, выдел€ющегос€ на электроде, меньше рассчитанного по уравнению ‘араде€, что св€зано с протекающими в электролизере побочными процессами. ќтношение массы вещества, выделившейс€ при электролизе на электроде, к теоретическому значению, рассчитанному по закону ‘араде€, называетс€ выходом по току (¬ѕ“, %).
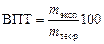 %, %,
| (8) |
где m теор Ц масса выдел€емого при электролизе вещества, рассчитанна€ по закону ‘араде€, m эксп Ц масса вещества, выделившегос€ в процессе эксперимента.
Ќапример, рассчитанное количество металла, выдел€ющегос€ на катоде, составило 6 г, а в ходе эксперимента было получено 4,8 г, соответственно выход по току составил 80 %.
2-й закон ‘араде€. ћассы прореагировавших на электродах веществ при посто€нном количестве электричества относ€тс€ друг к другу как мол€рные массы их эквивалентов:
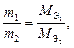
| (9) |
где m 1, MЁ 1 Ц масса и мол€рна€ эквивалентна€ масса вещества, выделившегос€ на одном электроде, а m 2, M Ё2 Ц на другом электроде.






