а) Pb + HNO3 конц →
б) S + HNO3 конц →
в) P + H2SO4 конц →
г) Mg + H2SO4 конц →
а) Металл + НNО3(конц) → соль + оксид азота + Н2О.
Формула оксида азота зависит от активности металла: N2O выделится, если в реакцию вступает активный металл (стоящий в ряду стандартных электродных потенциалов в интервале Li...Al);
NО выделится, если в реакцию вступает металл средней активности (Mn–Рb);
NO2 выделится, если в реакцию вступает малоактивный металл (стоящий в ряду стандартных электродных потенциалов после водорода).
| Дано: а) Pb + HNO3 конц → S + HNO3 конц → б) P + H2SO4 конц → Mg + H2SO4 конц → | Решение а) Pb0 + HN+5O3 конц = восст. окисл. = Pb+2(NO3)2 + N+2O + H2O НОК ДМ восстановитель Pb0 – 2ē = Pb+2 3 окислитель N+5 + 3ē = N+2 2 3Pb0 + 2N+5 = 3Pb+2 + 2N+2 |
| Уравнять реакции и указать окислитель и восстановитель |
Переносим полученные коэффициенты в молекулярное урав-нение:
3Pb0 + 2HN+5O3(конц) = 3Pb+2(N+5O3)2 + N+2O + H2O.
Поскольку азотная кислота расходуется не только на получение 2 моль NO, но и на получение 3 моль Pb(NO3)2, в которых содержится 6NO  со степенью окисления N+5, то для протекания этого процесса необходимо дополнительно 6 моль HNO3:
со степенью окисления N+5, то для протекания этого процесса необходимо дополнительно 6 моль HNO3:
 6HNO3 (конц) + 3Pb0 + 2HNO3(конц) = 3Pb(NO3)2 + 2NO + H2O
6HNO3 (конц) + 3Pb0 + 2HNO3(конц) = 3Pb(NO3)2 + 2NO + H2O
Суммируем число моль HNO3 и уравниваем количество водорода и кислорода (4Н2О):
3Рb + 8HNO3(конц) = 3Pb(NO3)2 + 2NO + 4Н2О.
б) Неметалл + HNO3(конц) → кислота, в которой неметалл проявляет высшую степень окисления + NO2 + (Н2О):
B → H3B+3O3; P → H3P+5O4; S → H2S+6O4; Se → H2Se+6O4;
Si → H2Si+4O3; C → H2C+4O3; As → H3As+5O4.
Решение
So + HN+5O3(конц) = Н2S+6O4 + N+4O2 + H2O
Восст. окисл.
НОК ДМ
восст-ль Sº – 6ē = S+6 1
окисл-ль N+5 + ē = N+4 6
Sº + 6N+5 = S+6 + 6N+4
S + 6HNO3(конц) = Н2SO4 + 6NO2 + 2H2O.
в) Неметалл + H2SO4(конц) → кислота, в которой неметалл проявляет высшую степень окисления + SO2 +(Н2O); см. пример б).
Решение
P0 + H2S+6O4(конц) = Н3Р+5О4 + S+4O2 + H2O
Восст. окисл.
НОК ДМ
восст-ль P0 – 5ē = P+5 2
окисл-ль S+6 + 2ē = S+4 5
2Р0 + 5S+6 = 2P+5 + 5S+4
2P + 5H2SO4(конц) = 2Н3РО4 + 5SO2 + 2H2O.
г) Металл + H2SO4(конц) → соль + (H2S, S, SO2)
(в зависимости от активности металла) + Н2О.
H2S выделится, если в реакцию вступает активный металл
(Li–Al),
S выделится, если в реакцию вступает металл средней активности (Mn–Рb),
SO2выделится, если в реакцию вступает малоактивный металл (стоящий в ряду стандартных электродных потенциалов после водорода).
Решение
Mg0 + H2S+6O4(конц) = Mg+2SO4 + H2S-2 + H2O.
восст. oкисл.
НОК ДМ
осст-ль Mg0 – 2ē = Mg+2 4
окисл-ль S+6 + 8ē = S-2 1
4Mg0 + S+6 = 4Mg+2 + S-2
Аналогично примеру (а) уравниваем реакцию:
4H2SO4(конц) + 4Mg + H2SO4(конц) = 4MgSО4 + H2S + 4H2O
4Mg + 5H2SO4(конц) = 4MgSО4 + H2S + 4H2O.
ГАЛЬВАНИЧЕСКИЕ ЭЛЕМЕНТЫ.
КОРРОЗИЯ МЕТАЛЛОВ.
УРОВЕНЬ В
1.а) Алюминиевый электрод погружен в 5∙10-4 М раствор сульфата алюминия. Вычислить значение электродного потенциала алюминия.
Дано:
Металл – Al
 = 5∙10-4 моль/л = 5∙10-4 моль/л
| Решение
Электродный потенциал алюминия рассчитываем по уравнению Нернста:
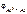 = =  + + 
|
 –? –?
|
По табл. 11.1 определяем стандартный электродный потенциал алюминия:
 = –1,67 В.
= –1,67 В.
Записываем уравнение электродного процесса, протекающего на поверхности алюминиевого электрода в растворе соли:
Al – 3ē = Al3+.
n – число электронов, участвующих в электродном процессе.
Для данной реакции n равно заряду иона алюминия Al3+(n = 3). Рассчитываем концентрацию ионов алюминия в растворе Al2(SO4)3:
 =
=  ∙ α ∙
∙ α ∙  .
.
Разбавленный раствор Al2(SO4)3 – сильный электролит.
Следовательно, α = 1. По уравнению диссоциации Al2(SO4)3.
Al2(SO4)3 = 2Al3+ + 3SO 
число ионов Al3+, образующихся при диссоциации одной молекулы Al2(SO4)3, равно 2.
Следовательно, 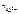 = 2.
= 2.
Тогда  = 5∙10-4∙1∙2 =
= 5∙10-4∙1∙2 =  моль/л.
моль/л.
Рассчитываем электродный потенциал алюминиевого электрода:
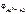 = -1,67 +
= -1,67 +  = –1,73 В.
= –1,73 В.
Ответ:  = –1,73 В.
= –1,73 В.
б) Потенциал цинкового электрода, погруженного в раствор своей соли, равен –0,75 В. Вычислить концентрацию ионов цинка в растворе.
Дано:
Металл – Zn
 = –0,75 В = –0,75 В
| Решение
Электродный потенциал цинка рассчитываем по уравнению Нернста:
 = =  + +  . .
|
 –? –?
|
Откуда:
 =
= 
По табл. 11.1 определяем стандартный электродный потенциал цинка:
 = 0,76 В, n – равно заряду иона цинка Zn2+ (n = 2).
= 0,76 В, n – равно заряду иона цинка Zn2+ (n = 2).
Тогда
 =
=  = 0,338.
= 0,338.
 = 100,339 моль/л = 2,18 моль/л
= 100,339 моль/л = 2,18 моль/л
Ответ:  = 2,18 моль/л.
= 2,18 моль/л.
2.Составить две схемы гальванических элементов (ГЭ), в одной из которых олово служило бы анодом, в другой – катодом. Для одной из них написать уравнения электродных процессов и суммарной токообразующей реакции. Вычислить значение стандартного напряжения ГЭ.
Решение
В гальваническом элементе анодом является более активный металл с меньшим алгебраическим значением электродного потенциала, катодом – менее активный металл с большим алгебраическим значением электродного потенциала.
По табл. 11.1 находим 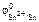 = –0,14 В.
= –0,14 В.






