Фурацилин
Furacilinum
Nitrofural *
O
||
O2N O CH = N – NH –C – NH2
5-нитрофурфуроласемикарбазон
Свойства
Подлинность
1.

O
|| + NaOH →
O 2 N O CH = N – NH – C – NH 2
→ O
||
O -N O CH - N = N –C – NH2
|
ONa

ONa
→ |
O2N O CH = N – N = C – NH2
2. а)
 to
to
O + 2 NaOH + H2O → NH3 ↑ + Na2CO3 +
||
O2N O CH = N – NH –C – NH2
 O
O
+ // + H2N – NH2
C - H
O2N OH
б)
3.
4.
5.
6.
7.
Количественное определение
1. Метод , способ
Метод основан на
Методика:

O + 2 I2 + 6 NaOH + → O +
|| //
O2N O CH = N – NH –C – NH2 O2N O C - H
моль • = экв.
+ N2 ↑ + NH3↑ + 4 NaI + Na2CO3 + 3 H2O
I2 + 2 NaOH → NaI + NaIO + H2O
NaI + NaIO + H2SO4 → I2 + Na2SO4 + H2O
I2 + 2 Na2S2O3 → 2 NaI + Na2S4O6
f экв . = M 1/ z =
2.
Применение
Наружно –
Внутрь –
Форма выпуска:
Хранение
Лекарственные средства, производные пиразола
Для медицины представляют интерес кето-произодные пиразолина и пиразолидина O
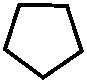
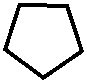 //
//
 1. 2.
1. 2.
N - H N - H
// //
O N O N
| |
H H
пиразолон -5 пиразолидиндион -3,5
К 1-ой группе относятся антипирин и анальгин, оказывающие жаропонижающее и болеутоляющее действие.
Ко 2-ой группе относится бутадион, оказывающий противовоспалительное действие.
Антипирин
Antipirynum
Phenazone *
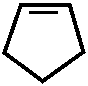 Н – - CH 3
Н – - CH 3
N – CH 3
//
O N
|
C 6 H 5
1-фенил-2,3-диметилпиразолон-5
Свойства
Подлинность
1.
3 Ant • 2 FeCl3
2.
2 NaNO2 + H2SO4 → 2 HNO2 + Na2SO4
 Н – -CH3 O = N – – CH3
Н – -CH3 O = N – – CH3
N –CH3 + HNO2 → N – CH3 + H2O
// //
O N O N
| |
C6H5 C6H5
3.
4.
Количественное определение
Метод , способ
Метод основан на
Методика:
 Н – -CH3 I – – CH3
Н – -CH3 I – – CH3
N –CH3 + I2 → N – CH3 + HI
// //
O N O N
| |
C6H5 C6H5
моль экв.
HI + CH3COONa → CH3COOH + NaI
I2 + 2 Na2S2O3 → 2 NaI + Na2S4O6
f экв. = M 1/ z =
Применение
Форма выпуска:
Хранение
Список
N. B.! При приготовлении ЛФ по рецептам необходимо учитывать способность антипирина реагировать с кислотами, фенолами, альдегидами, солями ртути, реагируя с которыми антипирин может переходить в жидкое состояние.
Анальгин
Analginum
Metamizol Sodium*
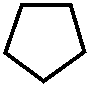 CH3 – N – -CH3
CH3 – N – -CH3
 /
/
NaO 3 S – CH 2 N – CH 3
// 1-фенил-2,3-диметил-4-метиламинопиразолон-
O N -5- N -метансульфонат натрия
|
C 6 H 5
Свойства
Подлинность
1. Ионы натрия –
2. Специфическая реакция на остаток метансульфоната натрия.
 CH3 – N – -CH3 CH3 – N – – CH3
CH3 – N – -CH3 CH3 – N – – CH3
/ to /
NaO3S – CH2 N –CH3 + HCl → H N – CH3 +
// //
O N O N
| |
C6H5 C6H5
О
//
+ SO2 ↑ + H – C = O ↑ + NaCl
Реакция на остаток формальдегида -
 O
O
HOOC – – H || H – – COOH H2SO4
+ H – C – H + →
HO – – OH
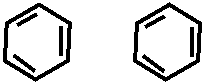 HOOC – – CH2 – – COOH H2SO4
HOOC – – CH2 – – COOH H2SO4
→ →
HO – – OH
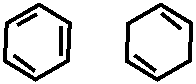 HOOC – – CH = – COOH
HOOC – – CH = – COOH
→
HO – = O
3.
4.
5.
Экспресс-анализ
1.
2.
Количественное определение
Метод , способ
Метод основан на
Методика:
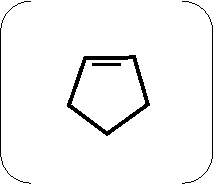 |
H
\ +
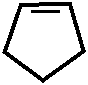 CH3 – N – -CH3 CH3 – N – – CH3
CH3 – N – -CH3 CH3 – N – – CH3
/ /
NaO3S – CH2 N –CH3 + I2 +2 H2O→ H N – CH3 I +
// //
O N O N
| |
C6H5 C6H5
моль экв.
О
//
+ NaHSO4 + H – C = O + HI
f экв. = M 1/ z =
Применение
Хранение
Бутадион
Butadionum
Phenylbutazone *
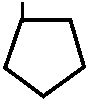 H
H
C4H9 – = O
N – C 6 H 5
// 1,2-дифенил -4-бутилпиразолидиндион- 3,5
O N
|
C 6 H 5
Свойства
 H
H
C 4 H 9 – = O C 4 H 9 – – OH
N – C 6 H 5 ↔ N – C 6 H 5
// //
O N O N
| |
C6H5 C6H5
 C4H9 – – OH C4H9 – – ONa
C4H9 – – OH C4H9 – – ONa
N – C6H5 + NaOH → N – C6H5 + H2O
// //
O N O N
| |
C 6 H 5 C 6 H 5
Подлинность
1.
 H
H
C4H9 – – O C4H9 – – ONa
N – C6H5 + NaOH → N – C6H5 + H2O
// //
O N O N
| |
C6H5 C6H5
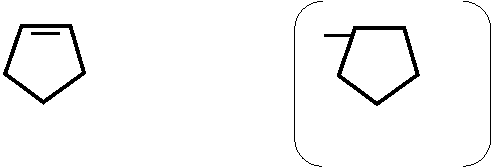
C4H9 – – ONa C4H9 – – O-
2 N – C6H5 + CuSO4 → N – C6H5 Cu ↓
// - Na2SO4 //
O N O N
| | 2
C6H5 C6H5
2.
 H
H
C4H9 – = O + H2SO4
+ NaNO2 [O]
N – C6H5 → NH – NH → N = N
// | | | |
O N C6H5 C6H5 C6H5 C6H5
|
C6H5
3.
4.
Экспресс-анализ
1.
2.
3.
Количественное определение
Метод , способ
Метод основан на
Методика:
 H
H
C4H9 – = O C4H9 – – OH
N – C6H5 ↔ N – C6H5
// //
O N O N
| |
C6H5 C6H5
 C4H9 – – OH C4H9 – – ONa
C4H9 – – OH C4H9 – – ONa
N – C6H5 + NaOH → N – C6H5 + H2O
// //
O N O N
| |
C6H5 C6H5
моль экв.
f экв. = M 1/ z =
Применение
Хранение






