ƒомашн€€ подготовка. ѕри изучении темы необходимо знать:
I) классификацию и номенклатуру неорганических соединений;
2) общие способы получени€ и химические свойства основных классов неор-ганических соединений Ц оксидов, гидроксидов †(основных, кислотных и ам-фотерных), солей (средних, кислых, основных); ††††††††††††††††††††††††††††††††††††††††††††††††††††3) правила составлени€ графических формул кислот, оснований и солей;†††††††††††††††††† 4) генетическую св€зь между классами неорганических соединений.
“аблица 2††††††††††††††††††††††††††††††††††††††††††
| Ќомер вариан-та | —оставьте формулы оксидов, гидрокси-дов, солей, докажите характер гидрокси-дов уравнени€ми реакций в молеку-л€рном и ионном виде | »сход€ из дан-ных соединений составьте форму-лы средних, кис-лых и основных солей. Ќазовите их.—оставьте гра-фические форму-лы. | †.¬ молекул€рном и ионном виде напишите уравнени€ реакций с по-мощью которых можно осуществить превращени€. |
| 1 | 2 | 3 | 4 |
| 1 | Cr+3; Pb+2; C+4 | H3PO4; Ca(OH)2 | Zn→ZnO→Na2ZnO2→Zn(OH)2→ →ZnSO4 |
| 2 | Be+2; Na+1; C+4 | Cr(OH)3; H2SO4 | AlCl3→Al(OH)3→NaAlO2→ →Al(OH)3 |
| 3 | Ca+2; Zn+2; S+6 | Al(OH)3; HNO3 | Al2O3→Al(NO3)3→AlOH(NO3)2→ →Al(OH)3 |
| 4 | N+5; Al+3; Ba+2 | Ba(OH)2; HNO3 | K→KOH→KCrO2→Cr(OH)3 |
| 5 | K+1; Cr+3; N+3 | Ca(OH)2; H2CO3 | FeCl2→Fe(OH)2→Fe(OH)3→Fe2O3→ →FeCl3 |
| 6 | Ca+2; Be+2; Cl+7 | Al(OH)3; †H2SO4 | AlCl3→Al(OH)3→NaAlO2→Al(OH)3 |
| 7 | Al+3; N+5; Cr+6 | H2CrO4; Ba(OH)2 | ZnSO4→Zn(OH)2→Na2ZnO2→ →Zn(OH)2 |
| 8 | S+4; Ni+2; Sn+2 | H2PbO2; Ba(OH)2 | Cr2(SO4)3→Cr(OH)3→NaCrO2→ →Cr(OH)3 |
| 9 | Mn+7; Cr+3; K+1 | Zn(OH)2; HNO3 | Cu→CuCl2→CuOHCl→Cu(OH)2→ →CuO |
| 10 | Si+4; Cd+2; Pb+2 | Al(OH)3; H2SO4 | Pb(NO3)2→Pb(OH)2→Na2PbO2→ →Pb(OH)2 |
| 11 | P+5; Fe+3; Cs+1 | H2SO3;Ba(OH)2 | Na→NaOH→Na2ZnO2→Zn(OH)2 |
| 12 | Se+6; Sr+2; Cl+1 | H3AsO4; KOH | Cr→CrCl3→Cr(OH)3→NaCrO2 |
| 13 | Pb+4; Mn+2; W+6 | Al(OH)3; H2SO4 | Al→NaAlO2→Al(OH)3→Al2O3 |
| 14 | Na+1; Mn+7; Cr+3 | Fe(OH)3; †H2SO4 | Zn →Na2ZnO2→Zn(OH)2→Zn(NO3)2 |
| 15 | S+6; Mg+2; Be+2 | Ba(OH)2; H2SO4 | BeSO4→Be(OH)2→K2BeO2→ →Be(OH)2 |
| 16 | Ag+1; Sn+2; S+4 | Cr(OH)3; LiOH | CaCl2→CaCO3→CaO→Ca(OH)2 |
| 17 | As+5; Ni+2; C+4 | Cu(OH)2; H2SO4 | Ca→Ca(OH)2→CaCO3→ →Ca(HCO3)2 |
| 18 | Fe+2; Pb+4; Si+4 | H3AsO4; KOH | Al→NaAlO2→Al(OH)3→Al2(SO4)3 |
| 19 | Cr+6; Hg+2; Zn+2 | Sb(OH)3; H2CrO4 | K→KOH→KCrO2→Cr(OH)3 |
| 20 | Cr+2; Si+4; Fe+3 | Ba(OH)2; H2S | MgCl2→Mg(OH)2→MgOHCl→ →MgCl2 |
ѕримеры выполнени€ задани€ 1
¬опрос 1
†
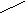 †††††† †††††††††††††Nа Ќ2–O4††††††††††††
†††††† †††††††††††††Nа Ќ2–O4††††††††††††
 ††††††††P+5 Ч ††P 2O5 Ч H3 –O4 ††Ч††††† Na3–O4
††††††††P+5 Ч ††P 2O5 Ч H3 –O4 ††Ч††††† Na3–O4
|
|
|
†††††††††††††††††† Nа2Ќ–O4†††††††††††
††P 2O5Ц кислотный оксид; оксид фосфора (V);
H3 –O4Ц кислотный гидроксид;ортофосфорна€ кислота;
Na 3 –O4 Ц средн€€ соль; фосфат натри€. ††
Nа 2 Ќ–O4 Ц кисла€ соль; гидрофосфат натри€. ††
Nа H2 –O4Ц кисла€ соль; дигидрофосфат натри€
†††††††††††††††††††††††††††††††††††††††††††† H3 –O4 + 3NaOH = Na3–O4 + 3H2O†††
†††††††††† †††††H3 –O4 + 3OH- = 3H2O + –O4 3-
†††††††††††††††††††††††††††††††††††††††††††† H3 –O4 ††+ HCl = реакци€ не идет
¬опрос 2
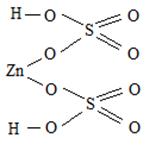
† ƒаны Zn (OH)2 и H 2 SO 4
Zn(OH)2 Ц двухкислотное основание Ц образует два р€да солей- средние и основные. —редн€€ соль ZnSO4, сульфат цинка; основна€ соль (ZnOH)2SO4, гидроксосульфат цинка. H2SO4 Ц двухосновна€ кислота, образует два р€да солей Ц средние и кислые, †кисла€ соль Zn(HSO4)2, гидросульфат цинка.
††††††††††††
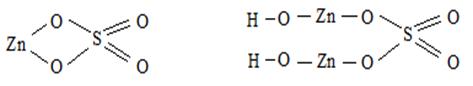
††††††††††††† 
††††††† †
¬опрос 3 ќсуществить превращен舆
†Al→Al(NO3)3→AlOH(NO3)2→ Al(OH)3 → Al2 (SO4)
†††††††††††††††††††††††††††† —r(NO3)3 + Al → Al(NO3)3 + —r
Al(NO3)3 + NaOH нед. →AlOH(NO3)2 + NaNO3
†††††††††††††††††† †Al3+ + OH- + 2NO3- †= AlOH(NO3)2
†††††††††††††††††††††††††† ††††AlOH(NO3)2 +2 NaOH= Al(OH)3 + 2NaNO3
††††††††††††††††††††† AlOH(NO3)2 + 2OH- = Al(OH)3 + 2NO3-
†††††††††††††††††††† 2Al(OH)3 +3H2SO4 = Al2 (SO4) 3 +6H2O
†††††††††††††††††††† 2Al(OH)3 + 6 H+ = 2 Al3+ + 6H2O
«адание 3. ’имическа€ термодинамика. инетика и химическое равновесие
ќбщие методические указани€. † †
ƒомашнее задание. ѕри выполнении заданий необходимо знать:
1) ѕервый и второй законы термодинамики
2) «акон √есса
3) акими факторами определ€етс€ величина теплового эффекта раст-ворени€;
4) как определ€етс€ стандартна€ теплота химической реакции;
5) что такое изобарно-изотермический потенциал, как он вычисл€етс€, при каких его значени€х реакци€ может протекать самопроизвольно;
†††† 6) ак определ€етс€ скорость химических реакций в гомогенных и ге-терогенных системах;
7) от каких факторов зависит скорость химических реакций;
8) закон действующих масс;
†† 9) правило ¬ант-√оффа;
† 10) принцип Ће Ўателье, как можно сместить химического равновеси€ в сторону получени€ целевого продукта.
–ешите следующие задачи:
1. –еакци€ образовани€ оксида азота (ΙV) выражаетс€ уравнение솆† 2NO+O2=2 NO2.
ак изменитс€ скорость пр€мой и обратной реакций, если давление уве-личить в 2 раза, а температуру оставить посто€нной? ¬ызовет ли это из-менение скоростей смещение равновеси€? (ќтвет: скорость пр€мой реак-ции увеличилась в 8 раз, скорость обратной реакции увеличилась в 4 раза).
2.¬о сколько раз изменитс€ скорость пр€мой и обратной реакции в сис-теме
2SO2(г)+ќ2(г)=2SO3(г),
если объем газовой смеси уменьшить в 3 раза? ¬ какую сторону сместитс€ равновесие системы? (ќтвет:в 27 раз;в 9 раз).
3. онстанта равновеси€ гомогенной системы CO(г)+Ќ2O(г)=CO2(г)+H2(г) при 850º— равна 1. ¬ычислите концентрации всех веществ при равновесии, если исходные концентрации: [CO]исх.=3 моль/л, [Ќ2ќ]исх.=2 моль/л. (ќтвет:[CO2]р=1,2 моль/л; [Ќ2]р=1,2 моль/л; [CO]р=1,8 моль/л; [Ќ2ќ]р=0,8 моль/л.
4.ѕри 393 реакци€ заканчиваетс€ за 10 мин. —колько времени будет продолжатьс€ реакци€ при 393 , если температурный коэффициент этой реакции равен 3? (ќтвет:4,5 ч.)
5.ѕри синтезе аммиака N2+3H2=2NH3 равновесие установилось при следующих концентраци€х реагирующих веществ: —N2=4 моль/л; —H2=2 моль/л; —NH3=6 моль/л. –ассчитайте константу равновеси€ этой реакции и ис-
|
|
|
ходные концентрации азота и водорода. (ќтвет: 1,1; —N2=7 моль/л; —H2= 11моль/л).
6. –еакци€ протекает по уравнению 4HCl+O2=2H2O+2Cl2. ¬ сторону ка- кой реакции сместитс€ химическое равновесие, если концентрации всех реагирующих веществ увеличить в 3 раза.
7. –еакци€ протекает по уравнению 4HCl+O2=2H2O+2Cl2. ¬ сторону какой реакции сместитс€ химическое равновесие, если концентрации всех реагиру-ющих веществ уменьшить в 2 раза.
†8. —корость некоторой реакции при 0 0— примем за единицу. ¬ычислите скорость той же реакции при 100 0—, если температурный коэффициент γ = 3.
9.”кажите, как повли€ет на равновесие в системе
—ќ(г) + Ќ2ќ(г) ↔ —ќ2(г) + Ќ2(г) ΔЌ = +42 кƒж:
а) увеличение температуры; б) уменьшение давлени€;
—оставьте выражение константы равновеси€ дл€ данного обратимого процесса.
10. ќпределите возможность протекани€ процесса при стандартных услови€х и при 1000 †
2NiO(к) + C(граф) = 2Ni(к) + CO2(г).
11. “емпературный коэффициент скорости некоторой реакции равен 2,3. ”кажите, как измен€етс€ скорость этой реакции при повышении температуры на 20 0—.
12. —оставьте выражение константы равновеси€ дл€ реакций:
а) ZnO(к) + —ќ(г) ↔ Zn (к) + CO2(г); ΔЌ >0;
б) 2—ќ(г) + 2Ќ2(г) ↔ —Ќ4(г) + —ќ2(г); ΔЌ< 0.
”кажите, как повли€ет на равновесие в этих системах:††† †††††††††††††††††††††††††††††††††††††††††а) уменьшение температуры; б) увеличение давлени€.
13. ѕользу€сь справочными данными, установите возможно ли при температурах 298 и 2500 протекание реакции:
“iO2(k) +2C (графит) = Ti (к) + 2—ќ (г).
14. »сход€ из теплоты образовани€ газообразного —ќ2 (ΔЌ = -393, 5††††††† кƒж/моль) и термохимического уравнени€:
—(графит) + 2 N2ќ (г) = —ќ2 †(г) + N2(г)†
¬ычислить теплоту образовани€ N2ќ.
15. ѕри взаимодействии газообразного метана и сероводорода образу-етс€ сероуглерод CS2 (г) и водород. Ќапишите термохимическое уравнение этой реакции и вычислите ее тепловой эффект. ќтвет: + 230,43 кƒж.
16. Ќа основании стандартных теплот образовани€ и абсолютных стандартных энтропий соответствующих веществ вычислите ΔGo298 реакции, протекающей по уравнению:
CO(г) + Ќ2ќ(ж) = —ќ2 †(г) + Ќ2(г).
¬озможна ли эта реакци€ при стандартных услови€х?
ќтвет: - 19,91 кƒж.
17. ѕри какой температуре наступит равновесие системы:
† 4HCl(г) + O2(г) ↔ Ќ2ќ(г) + 2Cl2 (г); ΔЌ = †-114,62 кƒж.
„то €вл€етс€ более сильным окислителем: хлор или кислород в этой системе, при каких температурах? ќтвет: 891 .
18. ќпределите ΔGo298 реакции, протекающей по уравнению:
4NH3(г) + 5O2(г) = 4NO(г) + 6 H2O(г).
¬ычислени€ сделайте на основании стандартных теплот образовани€ и абсолютных стандартных энтропий соответствующих веществ. ¬озможна ли эта реакци€ при стандартных услови€х? ќтвет: - 957,77 кƒж.
19.ќбразование сероводорода из простых веществ протекает по урав-нению:
††††††† †††H2(г) + S(ромб) = H2S(г); ΔЌ = - 20,15 кƒж.†††††††††††††††††††††††††††††††††††††††††††
»сход€ из значений So298 cоответствующих веществ, вычислите ΔSo298 и ΔGo298 этой реакции. ¬озможно ли образование H2S из простых веществ при стандартных услови€х? ќтвет: 43,15 кƒж/моль∙град; - 33,01 кƒж.
†††† 20.„ем можно объ€снить, что при стандартных услови€х возможна экзотермическа€ реакци€, протекающа€ по уравнению:
††††††††††††††††† H2(г) + CO2(г) = CO(г) + H2O(ж); ΔЌ = - 2,85 кƒж.
¬ывод сделайте на основании качественного изменени€ энтропии. «на€ тепловой эффект реакции и абсолютные стандартные энтропии соответствующих веществ, определите ΔGo298 этой реакции.
ќтвет: +19,91 кƒж.
†† †задание 4†






