††† —тепенью окислени€ атома элемента в соединении называетс€ условный зар€д, вычисл€емый по определенным правилам с учетом электроотрицательности атомов и смещени€ электронной плотности при образовании химической св€зи. ¬ дальнейшем степень окислени€ атома будем указывать по системе знак-число, а зар€д иона Ц по системе число-знак.
ѕравила вычислени€ степеней окислени€:
††† 1) степень окислени€ атома фтора в любых соединени€х всегда -1,
††† 2) степени окислени€ атомов щелочных металлов в соединени€х всегда +1,
††† 3) степень окислени€ атома водорода в соединени€х +1, а в гидридах металлов она равна -1, например: LiH, CaH2;
††† 4) степень окислени€ атома кислорода обычно равна -2, кроме пероксидов (Na2O2Ц1), надпероксидов (KO2Ц1/2), озонидов (KO3Ц1/3) и соединений кислорода со фтором, где атомы кислорода имеют положительные степени окислени€;
††† 5) алгебраическа€ сумма степеней окислени€ всех атомов в молекуле равна нулю, а в ионе Ц зар€ду иона.
††† ѕор€док "старшинства" названных правил возрастает с уменьшением указанного номера правила.
††† ’имические реакции, в которых происходит изменение степеней окислени€ атомов в молекулах, называютс€ окислительно-восстановитель-ными реакци€ми (ќ¬–). ¬ещества, молекулы которых принимают электроны, называютс€ окислител€ми. ¬ химической реакции такие вещества восстанавливаютс€. ¬ещества, молекулы которых отдают электроны, называютс€ восстановител€ми. ¬ течение реакции такие вещества окисл€ютс€.
“ипичными окислител€ми €вл€ютс€:
††† 1) вещества, молекулы которых содержат атомы элементов в высших положительных степен€х окислени€, например: KMn+7O4, KBi+5O3, K2Cr+62O7, Pb+4O2;
††† 2) катионы металлов более высокого зар€да (более высокой степени окислени€), например: Fe3+, Au3+, Sn4+;
††† 3) галогены и кислород (при повышенных температурах).
“ипичными восстановител€ми €вл€ютс€:
††† 1) вещества, молекулы которых содержат атомы элементов в высоких отрицательных степен€х окислени€ или степени окислени€ которых легко повышаютс€, например: Na2SЦ2, KIЦ1, NЦ3H3, KN+3O2, K2S+4O3;
††† 2) катионы металлов более низкой степени окислени€, например: Fe2+, Sn2+;
††† 3) металлы, из них в первую очередь Ц щелочные и щелочно-земельные металлы, а также Ц водород при повышенных температурах.
ќбычно различают следующие основные типы ќ¬–:
††† 1) межмолекул€рные ќ¬– Ц в этих реакци€х окислителем и восстановителем €вл€ютс€ разные молекулы, например:
2KMn+7O4 + 10KIЦ1 + 8H2SO4 = 2Mn+2SO4 + 6K2SO4 + 5I2 + 8H2O
†††††††††††††††††††††††
†††††††††††† Mn+7 + 5ē = Mn+2†††† | 2 (окислитель)
†††††††††††††††††††††††††††††††† 2IЦ Ц 2ē = I2††††††††††††† | 5 (восстановитель)
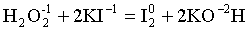
†††††††††††††††††††††††††††††††† 2OЦ1 + 2ē = 2OЦ2 | 1 (окислитель)
†††††††††††††††††††††††††††††††† 2IЦ Ц 2ē = I2††††††††††††† | 1 (восстановитель)
††† 2) внутримолекул€рные ќ¬– Ц в этих реакци€х окислителем и восстановителем €вл€ютс€ атомы различных или одинаковых элементов, наход€щихс€ в разных част€х одной молекулы, например:
|
|
|
t
†††† (NЦ3H4)2Cr+62O7 = N02 + Cr+32O3 + 4H2O
†††††††††††††††††††††††††††††††† 2NЦ3 Ц 6ē = 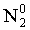 ††| 1 (восстановитель)
††| 1 (восстановитель)
†††††††††††††††††††††††††††††††† 2Cr6+ + 6ē = 2Cr3+ | 1 (окислитель)
250oC
†NЦ3H4N+5O3 = N+12O + 2H2O
†††††††††††††††††††††††††††††††† N+5 + 4ē = N+1 | 1 (окислитель)
†††††††††††††††††††††††††††††††† NЦ3 Ц 4ē = N+1 | 1 (восстановитель)
†††††† 3) реакции диспропорционировани€, в которых окислителем и восстановителем €вл€ютс€ одни и те же атомы в молекуле, например:
t
†††††††††††† 3Cl02 + 6KOH = 5KClЦ1 + KCl+5O3 + 3H2O
†††††††††††††††††††††††††††††††† 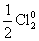 †+ ē = ClЦ | 5
†+ ē = ClЦ | 5
†††††††††††††††††††††††††††††††† 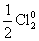 †Ц 5ē = Cl+5 | 1
†Ц 5ē = Cl+5 | 1
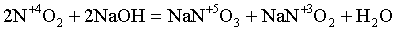
†††††††††††††††††††††††††††††††† N+4 + ē = N+3† | 1
†††††††††††††††††††††††††††††††† N+4 Ц ē = N+5†† | 1
††† –ассмотрим поведение наиболее распространенных окислителей и восстановителей в ќ¬–.
††† јзотна€ кислота, €вл€€сь окислителем, восстанавливаетс€ обычно до оксидов азота 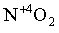 ,
, 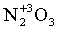 ,
, 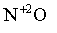 ,
, 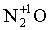 или, в случае сильных восстановителей Ц до
или, в случае сильных восстановителей Ц до 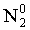 †или иона аммони€ N-3H4+. ќтметим, что образование N2O3 происходит редко и этот случай можно не рассматривать.
†или иона аммони€ N-3H4+. ќтметим, что образование N2O3 происходит редко и этот случай можно не рассматривать.
††† √лубина восстановлени€ N+5 из азотной кислоты одинаковой концентрации возрастает с увеличением силы восстановител€. ѕри одном и том же восстановителе глубина ее восстановлени€ увеличиваетс€ с уменьшением концентрации кислоты. »ллюстрируем эти закономерности на примерах взаимодействи€ азотной кислоты с металлами.
††† ѕри этом полезно помнить, что взаимодействие меди с азотной кислотой различной концентрации описываетс€ уравнени€ми:
Cu0 + 4HN+5O3(конц) = Cu+2(NO3)2 + 2N+4O2 + 2H2O
†††††††††††††††††††††††††††††††† Cu0 Ц 2ē = Cu+2 | 1
†††††††††††††††††††††††††††††††† N+5 + ē = N+4† | 2
3Cu0 + 8HN+5O3(разб) = 3Cu+2(NO3)2 + 2N+2O + 4H2O
†††††††††††††††††††††††††††††††† Cu0 Ц 2ē = Cu+2 | 3
†††††††††††††††††††††††††††††††† N+5 + 3ē = N+2 | 2
††† –азумеетс€, эти и последующие уравнени€ реакций различных веществ с азотной кислотой по составу продуктов соответствуют начальной стадии процессов, по мере их протекани€ и уменьшени€ концентрации кислоты-окислител€ неизбежно мен€етс€ состав продуктов восстановлени€ HNO3.
††† ѕродукты же восстановлени€ азотной кислоты другими металлами будут определ€тьс€ положением этих металлов в р€ду активности по отношению к меди или, говор€ более строго, величинами стандартных электродных потенциалов. “ак, например, при взаимодействии с Cu, Zn, Mg и состав продуктов восстановлени€ кислоты будет примерно следующим:
††††††††††††††††††††††††††††††††††††††††† Cu†††† NO2
†††††††††††††††††††††† HNO3(конц) + Zn ††† Ѓ NO (N2O)
††††††††††††††††††††††††††††††††††††††††† Mg††††††††††††† N2O (N2)
††††††††††††††††††††††††††††††††††††††††† K†††††† N2 (NH4+)
††††††††††††††††††††††††††††††††††††††††† Cu†††† NO
†††††††††††††††††††††† HNO3(разб) + Zn ††† Ѓ N2O (N2)
††††††††††††††††††††††††††††††††††††††††† Mg††††††††††††† N2 (NH4+)
††††††††††††††††††††††††††††††††††††††††† K†††††† NH4+
††† — неметаллами реально взаимодействует лишь концентрированна€ азотна€ кислота, котора€ при этом, как правило, восстанавливаетс€ до NO2 или до NO. ѕри этом неметалл окисл€етс€ до высшей положительной степени окислени€ в форме кислоты, если она устойчива, или в форме оксида, если кислота неустойчива, например:
|
|
|
††††††††††††††††††††††††††††††††††††††††† P4†††† H3P+5O4
†††††††††††††††††††††† HNO3(конц) + S Ѓ H2S+6O4
††††††††††††††††††††††††††††††††††††††††† C††††† C+4O2
††††††††††††††††††††††††††††††††††††††††† B††††† H3B+3O3
††† ќкислительное действие перманганата кали€ в зависимости от кислотности среды описываетс€ следующей схемой:
††††††††††††††††††††††††††††††††††††††††† Ѓ +5ē, H+Ѓ Mn+2 (MnSO4)
†††††††††††††††††††††† KMnO4 Ѓ +3ē, H2O Ѓ †††† Mn+4 (MnO2)
††††††††††††††††††††††††††††††††††††††††† Ѓ +ē, OHЦЃ Mn+6 (K2MnO4)
††† ѕерманганат кали€ €вл€етс€ сравнительно сильным окислителем в кислой среде, окислителем средней силы Ц в нейтральной среде, в щелочной среде представл€ет собой относительно слабый окислитель. ѕримеры ќ¬– с участием ћnO4:
2KMn+7O4 + 5KN+3O2 + 3H2SO4 = 2Mn+2SO4 + 5KN+5O3 + K2SO4 + 3H2O
†††††††††††††††††††††††††††††††† Mn+7 + 5ē = Mn+2†††† | 2
†††††††††††††††††††††††††††††††† N+3 Ц 2ē = N+5 | 5
3KMn+7O4 + 3CЦ2H3OH 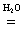 †3HC0HO + 2Mn+4O2 + 2KOH + 2H2O
†3HC0HO + 2Mn+4O2 + 2KOH + 2H2O
†††††††††††††††††††††††††††††††† Mn+7 + 3ē = Mn+4†††† | 2
†††††††††††††††††††††††††††††††† CЦ2 Ц 2ē = C0††††††† | 3
2KMn+7O4 + 2KIЦ1 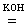 2K2Mn+6O4 + I20
2K2Mn+6O4 + I20
†††††††††††††††††††††††††††††††† Mn+7 + ē = Mn+6 | 2
†††††††††††††††††††††††††††††††† 2IЦ1 Ц 2ē = I20† | 1
††† ќбратим внимание, что в двух последних случа€х вода и едкое кали не €вл€ютс€ непосредственными участниками ќ¬–, однако они должны быть указаны над равенством, чтобы показать, в какой среде протекает химическа€ реакци€. ќтметим также, что приведенна€ выше схема восстановлени€ ћnO4 отражает одновременно и наиболее стабильные в той или иной среде формы соединений.
††† —ерна€ кислота в разбавленном растворе €вл€етс€ окислителем за счет ионов Ќ+, в концентрированном, гор€чем растворе (более 50 мас. % H2SO4) серна€ кислота представл€ет собой сравнительно сильный окислитель, при этом в качестве окислител€ выступает S+6. ¬ случае слабых восстановителей S+6 восстанавливаетс€ до S+4, как правило, в виде SO2, например:
2H2S+6O4(конц) + Cu0 = Cu+2SO4 + S+4O2 + 2H2O
†††††††††††††††††††††††††††††††† Cu0 Ц 2ē = Cu+2 | 1
†††††††††††††††††††††††††††††††† S+6 †+ 2ē = S+4 | 1
2H2S+6O4(конц) + S0 = 3S+4O2 + 2H2O
†††††††††††††††††††††††††††††††† S+6 †+ 2ē = S+4 | 2
†††††††††††††††††††††††††††††††† S0 †Ц 4ē = S+4†† | 1
2H2SO4(конц) + 2KBr (кр) = K2SO4 + Br2 +SO2 + 2H2O
†††††††††††††††††††††††††††††††† S+6 †+ 2ē = S+4 | 1
†††††††††††††††††††††††††††††††† 2BrЦ Ц 2ē = Br20 | 1
††† ѕри наличии сильного восстановител€ восстановление S+6 идет более глубоко Ц до S0 или даже до Ќ2SЦ2, например:
4H2S+6O4(конц) + 6KIЦ1(кр) = S0 +3I20 + 3K2SO4 + 4H2O
†††††††††††††††††††††††††††††††† S+6 †+ 6ē = S0† | 1
†††††††††††††††††††††††††††††††† 2IЦ Ц 2ē = I20†††††††††††† | 3
5H2SO4(конц) + Mg0 = 4Mg+2SO4 + H2S-2 + 4H2O
†††††††††††††††††††††††††††††††† S+6 †+ 8ē = SЦ2 | 1
†††††††††††††††††††††††††††††††† Mg0 Ц 2ē = Mg+2 | 4
††† Ўироко распространенным и удобным окислителем €вл€етс€ кислый раствор бихромата кали€, Cr+6 в этом соединении восстанавливаетс€ до Cr+3 в форме солей:
K2Cr+62O7 + 3KN+3O2 + 4H2SO4 = Cr+32(SO4)3 + 3KN+5O3 + K2SO4 + 4H2O
†††††††††††††††††††††††††††††††† 2Cr+6 †+ 6ē = 2Cr+3†† | 1
†††††††††††††††††††††††††††††††† N+3 Ц 2ē = N+5 | 3
K2Cr+62O7+3C-1H3C-1HO+4H2SO4 = Cr+32(SO4)3 + 3C0H3C0OOH+K2SO4+4H2O
†††††††††††††††††††††††††††††††† 2Cr+6 †+ 6ē = 2Cr+3†† | 1
|
|
|
†††††††††††††††††††††††††††††††† 2CЦ1 Ц 2ē = 2C0 | 3
††† ¬ щелочной среде бихроматы быстро переход€т в хроматы в соответствии с уравнением:
Cr2O72Ц + 2OHЦ = 2CrO42Ц + H2O,
а в кислой среде, наоборот, хроматы легко переход€т в бихроматы:
2CrO42Ц + 2H+ = Cr2O72Ц + H2O.
††† Ёто обсто€тельство следует учитывать при написании уравнений реакций с участием соединений Cr+6. ќбратим внимание, что соединени€ Cr+6 в качестве окислителей целесообразно использовать лишь в кислой среде, в щелочной среде они представл€ют собой крайне слабые окислители; эти обсто€тельства достаточно красноречиво иллюстрируютс€ соответствующими величинами стандартных электродных потенциалов:
Cr2O72- + 14H+ + 6ē = 2Cr+3 + 7H2O, E0 = 1,33 B
CrO42- + 4H2O + 3ē = Cr(OH)3 + 5OHЦ, E0 = Ц0,13 B
††† ѕоскольку в молекуле пероксида водорода атомы кислорода имеют промежуточную степень окислени€ (-1), то молекулы этого соединени€ могут быть как окислител€ми, так и восстановител€ми. ќбычно в качестве окислител€ используют водные растворы с концентрацией этого соединени€ более 10Ц15 мас.%, а разбавленные растворы (1Ц5 мас.% H2O2) Ц примен€ют в качестве восстановител€:
H2O2Ц1 + 2KIЦ1 + H2SO4 = I20 +2H2OЦ2 + K2SO4
†††††††††††††††††††††††††††††††† 2OЦ1 †+ 2ē = 2OЦ2 | 1
†††††††††††††††††††††††††††††††† 2IЦ1 Ц 2ē = I20† | 1
5H2O2Ц1 + 2KMn+7O4 + 3H2SO4 = 2Mn+2SO4 + K2SO4 + 5O20 + 8H2O
†††††††††††††††††††††††††††††††† 2OЦ1 †+ 2ē = O20 | 5
†††††††††††††††††††††††††††††††† Mn+7 + 5ē = Mn+2†††† | 2
††† ’лорна€ и бромна€ вода представл€ют собой разбавленные растворы соответствующих галогенов (растворимость Cl2 и Br2 невелика): окисл€€ какие-либо соединени€, галогены восстанавливаютс€ до анионов √ Ц1:
††††††††††††† Cl20 + 2ē = 2ClЦ1††††††††††††††† Br20 + 2ē = 2BrЦ1
††† Ќаиболее распространенными в лабораторной практике €вл€ютс€ следующие восстановители: нитриты (например KN+3O2), сульфиды (K2SЦ2), сульфиты (K2S+4O3), иодиды (KIЦ1), гипофосфиты (KH2P+1O2), фосфиты (K2HP+3O3), соединени€ олова (II) (SnCl2), железа (II) (FeSO4), магний, алюминий, цинк. ¬ реакци€х окислени€-восстановлени€ указанные вещества окисл€ютс€, как правило, по следующим схемам:
N+3O2Ц Ц 2ē Ѓ N+5O3Ц
SЦ2 Ц 2ē = S0
S+4O32Ц Ц 2ē Ѓ S+6O42Ц
2IЦ1 Ц 2ē = I20
H2P+1O2Ц Ц 2ē Ѓ H2P+3O3Цили
H2P+1O2Ц Ц 4ē Ѓ H2P+5O4Ц
Sn+2 Ц 2ē = Sn+4
Fe2+ Ц ē = Fe3+
††† ћеталлы окисл€ютс€ до соответствующих катионов. ѕолезно помнить, что в случае амфотерных металлов в разбавленных водных растворах при наличии избытка щелочи формой существовани€ соединений будут, как правило, гидроксокомплексы, например: K3[Al(OH)6], Na2[Zn(OH)4].
††† ¬ неорганической химии существуют своеобразные универсальные окислитель и восстановитель Ц так называемые "щелочной плав" и "водород
в момент выделени€". ѕервый представл€ет собой композицию из кристаллического соединени€, разлагающегос€ при нагревании с выделением кислорода (например 2O2, KNO3, KClO3 и т.п.) и щелочного компонента. "ўелочной плав" в большинстве случаев окисл€ет атомы элементов соответствующих соединений до высших положительных или близких к ним степеней окислени€, например:
3KN+5O3 + W + 2KOH 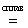 3KN+3O2 + K2W+6O4 + H2O
3KN+3O2 + K2W+6O4 + H2O
†††††††††††††††††††††††††††††††† N+5 †+ 2ē = N+3 | 3
†††††††††††††††††††††††††††††††† W0 Ц 6ē = W+6 | 1
KCl+5O3 + 3Mn+4O2 + 6KOH 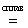 KClЦ1 + 3K2Mn+6O4 + 3H2O
KClЦ1 + 3K2Mn+6O4 + 3H2O
|
|
|
†††††††††††††††††††††††††††††††† Mn+4 †Ц 2ē = Mn+6††† | 3
†††††††††††††††††††††††††††††††† Cl+5 + 6ē = ClЦ1 | 1
††† ƒействующим началом "щелочного плава" €вл€етс€ атомарный (молекул€рный) кислород, получающийс€ при разложении соответствующего соединени€.
††† –еакци€ между кусочками металла, погруженного в минеральную кислоту, и раствором или кусочками амфотерного металла, наход€щегос€ в растворе щелочи, идет на поверхности металла. »менно там восстанавливаютс€ протоны и образуютс€ атомы (молекулы) водорода. ≈сли в такой системе находитс€ соединение, способное восстанавливатьс€, то оно и будет восстановлено до высшей отрицательной или близкой к ней степени окислени€ атома (атомов) элемента. атионы металлов будут восстанавливатьс€ в этих услови€х до минимальной положительной степени окислени€. ќписанна€ восстановительна€ система и представл€ет собой "водород в момент выделени€". ѕример:
3Zn0 + 9HCl + Na3As+3O3 = AsЦ3H3 + 3Zn+2Cl2 + 3NaCl + 3H2O
†††††††††††††††††††††††††††††††† Zn0 †Ц 2ē = Zn+2 | 3
†††††††††††††††††††††††††††††††† As+3 + 6ē = AsЦ3 | 1
††† » "щелочной плав", и "водород в момент выделени€" активно используютс€ при проведении ќ¬–, первый особенно удобен при переведении металлов, не раствор€ющихс€ в обычных минеральных кислотах, в растворимые соли; второй Ц эффективен при получении водородных соединений неметаллов. –азумеетс€, не следует забывать, что абсолютным окислителем €вл€етс€ электрический ток на аноде, а абсолютный восстановитель Ц электрический ток на катоде.






