¬ химическом анализе приходитс€ иметь дело с измерени€ми количеств твердых веществ и концентраций растворов. ’орошо известно, что атомы и молекулы взаимодействуют в определенных пропорци€х. Ќо непосредственно подсчитывать число атомов или молекул, участвующих в реакции, было бы неудобно. ƒл€ упрощени€ расчетов в химии было введено пон€тие моль. ќдин моль атомов, молекул, ионов или других частиц содержит количество частиц, равное числу јвогадро (6,022Ј1023).
ћоль - количество вещества (n), в котором содержитс€ число структурных частиц (молекул, атомов, ионов и др.), равное посто€нной јвогадро (6,02-1023). ѕри использовании мол€ как единицы количества вещества следует указывать вид частиц. Ќапример: n (NaOH) = 2 моль - количество вещества, содержащего 2Ј6,02Ј1023 молекул NaOH; n (—а2+) = 0,5 моль - количество вещества, содержащего 0,5Ј6,02Ј1023 ионов —а2+.
ћасса одного мол€ вещества называетс€ мол€рной массой. ќтсюда, масса вещества равна произведению количества вещества на мол€рную массу.
m = ν ЈM
¬ химии большое практическое значение имеют такие характеристики элементов (дл€ атомов) или веществ (дл€ молекул), как эквиваленты, или мол€рна€ масса эквивалента.
–асчет результатов основан на принципе эквивалентности, в соответствии с которым вещества реагируют между собой в эквивалентных количествах.
≈сли определ€емое вещество A реагирует с вееством B по уравнению
νAA + νBB → ѕродукты реакции,
то эквивалентными массами веществ будут νA M (A) и νB M (B), где M (A) и M (B) Ц мол€рные массы веществ A и B, а νA и νB Ц стехиометрические коэффициенты.
”равнению реакции можно придать вид
A + (νB/νA)B → ѕродукты реакции,
где νA>νB, что означает, что одна частица вещества A будет эквивалентна νB/νA частиц вещества B.
ќтношение νB/νA обозначают символом f э и называют фактором эквивалентности вещества B
fэ (B) = νB/νA..
‘актор эквивалентности €вл€етс€ безразмерной величиной, равной или меньшей единицы.
¬еличину νB/νAB или равную ей fэ (B)B называют эквивалентом вещества B.
¬о избежание противоречий необходимо приводить реакции к единой общей основе. ƒл€ реакций кислотно-основного взаимодействи€ такой основой может быть ион водорода.
¬ окислительно-восстановительных реакци€х количество реагирующего вещества удобно св€зывать с числом электронов, принимаемых или отдаваемых данным веществом. Ёто позвол€ет дать следующее определение.
Ёквивалентом называетс€ реальна€ или условна€ частица, котора€ может присоедин€ть, высвобождать или быть каким-либо другим образом эквивалентна одному иону водорода в кислотно-основных реакци€х или одному электрону в окислительно-восстановительных реакци€х.
ѕри использовании термина "эквивалент" всегда необходимо указывать, к какой конкретной реакции он относитс€.
ѕод условной частицей понимаютс€ как реально существующие частицы (молекулы, ионы, электроны и т.д.), так и доли таких частиц (например,1/2 иона) или их группы.
|
|
|
≈диницей количества вещества эквивалента €вл€етс€ моль. Ќапример, в реакции
NaOH + 1/2 H2SO4 → 1/2 Na2SO4 + H2O
f э(NaOH)=1; f э(H2SO4)=1/2
Ёквивалент серной кислоты в этой реакции будет составл€ть половину молекулы (условна€ частица)
f э(H2SO4) H2SO4 = 1/2 H2SO4
ƒл€ реакции
H3PO4 + KOH → KH2PO4 + H2O
f э(H3PO4)=1; f э(H3PO4) H3PO4 = H3PO4,
а дл€ реакции
H3PO4 + 3 KOH → K3PO4 + 3H2O
f э(H3PO4)=1/3; f э(H3PO4) H3PO4 = 1/3 H3PO4.
¬ полуреакции
MnO4- + 8H+ + 5e → Mn2+ + 4 H2O
f э(KMnO4)=1/5; f э(KMnO4) KMnO4 = 1/5 KMnO4,
но в полуреакции
MnO4- + 4H+ + 3e → MnO2 + 2H2O
f э(KMnO4)=1/3; f э(KMnO4) KMnO4 = 1/3 KMnO4.
‘актор эквивалентности и эквивалент данного вещества €вл€ютс€ не посто€нными величинами, а завис€т от стехиометрии реакции, в которой они принимают участие. “аким образом,
‘актор эквивалентности Ц это число, обозначающее, кака€ дол€ реальной частицы вещества X эквивалентна одному иону водорода в данной кислотно-основной реакции или одному электрону в реакции окислени€-восстановлени€.
ћол€рной массой эквивалента (M э) вещества X называют массу одного мол€ эквивалента этого вещества, равную произведению фактора эквивалентности на мол€рную массу вещества X. ƒл€ мол€рной массы эквивалента в литературе встречаетс€ также термин "эквивалентна€ масса".
ћол€рный объем - объем, занимаемый мол€рной массой газообразного вещества. ћол€рный объем любого газа при нормальных услови€х равен 22,4 л.
Ёквивалентный объем - объем, занимаемый мол€рной массой эквивалента газообразного вещества. 1 моль водорода содержит 2 эквивалента. ќбъем мол€рной массы эквивалента водорода (1 г/моль) - 22,4/2 = 11,2 л. 1 моль кислорода содержит 4 эквивалента. ќбъем мол€рной массы эквивалента кислорода (8г/моль) 22,4/4 = 5,6 л.
онцентрации растворов выражают множеством способов. ћол€рность или мол€рна€ концентраци€ Ц сама€ распространенна€ единица измерени€ концентрации. ѕо определению одномол€рным раствором называетс€ такой раствор, в одном литре которого содержитс€ один моль вещества. ¬ более общем случае мол€рностью раствора называетс€ его концентраци€, выраженна€ в виде количества молей в литре (моль/л).

¬место обозначени€ моль/л часто используют ћ, например, одномол€рный раствор можно записать 1 моль/л или 1 ћ. ¬ экспериментах часто имеют дело с достаточно малыми количествами вещества, дл€ которых удобнее использовать единицы измерени€ миллимоль, милиграмм, милилитр. ќбратите внимание, что единица г/моль Ц это то же самое, что мг/ммоль; г/л Ц то же самое, что мг/мл; а моль/л Ц то же самое, что ммоль/мл.
ƒл€ многих достаточно концентрированных коммерческих растворов кислот и щелочей в качестве концентрации указываетс€ массова€ дол€ растворенного вещества (в процентах). ќна рассчитываетс€, как известно, следующим образом.

„асто возникает необходимость приготовить из концентрированных препаратов, растворы заданной мол€рности. ≈сли дана мол€рна€ концентраци€ исходного раствора, то найти мол€рную концентрацию раствора, полученного его разбавлением очень легко. ѕри разбавлении вещества его количество не измен€етс€, лишь увеличиваетс€ объем раствора и уменьшаетс€ концентраци€.
ν = CЈV
ќтсюда следует, что произведение мол€рной концентрации раствора на его объем есть величина посто€нна€.
|
|
|
C 1 ЈV 1 = C 2 ЈV 2
где C 1и C 2ЈЦ мол€рные концентрации исходного и конечного растворов; V 1и ЈV 2 Ц объемы исходного и конечного растворов.
¬ случае использовани€ исходных растворов, дл€ которых указана массова€ дол€, вычислени€ провод€тс€ следующим способом.

ќбъем раствора св€зан с массой раствора простым соотношением:

ќбъедин€€ формулы получаем:
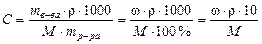
„тобы выполнить соответствующие расчеты, необходимо знать плотность исходного раствора. Ќапомним, что плотностью называетс€ масса единицы объема вещества при заданной температуре (обычно 20 ∞—), выражаема€, как правило, в г/мл (г/см3).
»ногда дл€ вещества указывают не плотность, а удельный вес. ”дельный вес Ц это отношение массы физического тела (например, раствора), наход€щегос€ при 20 ∞—, к массе равного объема воды, наход€щегос€ при 4 ∞— (иногда 20 ∞—). “аким образом, удельный вес Ц это отношение плотностей двух веществ, т.е. величина безразмерна€. ѕоскольку плотность воды при 4 ∞— составл€ет 1,00000 г/мл, то плотность вещества равна его удельному весу, отнесенному к воде при 4 ∞—. “.е. при проведении вычислений значение удельного веса можно использовать вместо плотности.
Ёквивалентность (нормальность) N показывает число эквивалентов растворенного вещества, содержащихс€ в 1 л раствора.
ћол€льность CM показывает количество растворенного вещества, содержащегос€ в 1000 г раствора.
ћол€рна€ дол€ ω ћ показывает долю в мол€х растворенного вещества по отношению к сумме молей всех компонентов, наход€щихс€ в растворе.
“итром “ называетс€ число грамм растворенного ещества, содержащихс€ в 1 см3 (мл) раствора.
ѕример 1. ќпределить, кака€ среда - кисла€, щелочна€ или нейтральна€ - получитс€ при смешивании 8 г NaOH с 10 г H2Sќ4.
–ешение.
‘ормула дл€ расчета: m1 /Ё1 = m2 /Ё2 применительно к задаче записываетс€ так: m (NaOH)/Ё(NaOH) = m (H2SO4)/Ё(H2SO4).
ѕодставим числовые значени€ Ё(NaOH) = 40;
Ё(H2SO4) = 49, получим: 8/40 < 10/49.
”читыва€, что
m (NaOH) / Ё(NaOH) = n Ё(NaOH); m (H2SO4)/ Ё (H2SO4)= n Ё(H2SO4),
делаем вывод: среда кисла€, так как дл€ реакции вз€то избыточное количество эквивалентов кислоты:
n Ё(NaOH) < n Ё(H2SO4).
ѕример 2. Ќайти мол€рную концентрацию раствора H2SO4, полученного разбавлением 5 мл раствора с концентрацией 1 моль/л в колбе на 1 л.
–ешение:
C 1 ЈV 1 = C 2 ЈV 2
¬ыражаем концентрацию конечного раствора и подставл€ем данные:

ѕереводить литры в миллилитры всегда не об€зательно, так как размерности все равно сокращаютс€, но главное сохран€ть единую размерность.
ѕример 3. Ќайти мол€рную концентрацию 15%-ного раствора H2SO4 (ρ = 1,10 г/мл).
–ешение:
ƒл€ вычислени€ мол€рной концентрации используем выведенную выше формулу:

ѕример 4. ¬ычислите: а) процентную ω; б)мол€рную C; в) мол€льную CM; г) эквивалентную N концентрацию раствора Ќ3–ќ4, соержащего 18 г кислоты в 282 см3 воды, если плотность его 1,031 г/ см3. „ему равен титр этого раствора?
–ешение.1) масса воды = 282 Ј1 = 282 г/ см3; 2) масса раствора = 18 + 282 = 300 г; 3) объем раствора = 300: 1,031=291 см3; 4) мол€рность раствора = (18/98): 0,291 =0,63 моль/л; 6)мол€льность раствора= [(18/98):282]Ј1000 = 0,65 моль/ 1000г Ќ2ќ; 7) эквивалентность N раствора =[(18/98:3)]: 0,291=1,89 моль/л; 8) титр раствора = 18/291 = 0,06 г/ см3.
ќЌ“–ќЋ№Ќџ≈ ¬ќѕ–ќ—џ » «јƒјЌ»я
111. —колько эквивалентов заключаетс€ в 1 моле —Ќз—ќќЌ, NH4OH, Na2O, ј1(ќЌ)3, A12(SO4)3, Ќ2, FeCl3, Ќ2O?
112. акова мол€рность мол€рной концентрации эквивалента раствора FeCl3 с 3 моль/л (3 н)?
113. ѕри взаимодействии кислорода и фосфора израсходовано 2,8 л кислорода (нормальные услови€). —колько эквивалентов –2O5 получилось?
114. »з 0,635 г оксида металла получено 0,4135 г металла. ќпределите мол€рную эквивалентную массу металла и оксида.
115. ƒл€ нейтрализации некоторого объема H2SO4 израсходовано 24,2 мл раствора мол€рной концентрации эквивалента щелочи - 0,1 моль/л. ¬ычислите, сколько H2SO4 было в растворе?
|
|
|
116. ќпределите мол€рную концентрацию и концентрацию раствора (—н) 30%-й Ќ—1 с р = 1,15г/мл. —колько этой кислоты потребуетс€ дл€ приготовлени€ 100 мл раствора концентрации 1 моль/л (1 н)?
117. —колько эквивалентов заключаетс€ в 1 моле ќ2, –2ќ5, Na2Cќ3, Ќ¬г, —о(ќЌ)2, —а3(–ќ4)2?
118. акова нормальность 1,5ћ раствора NiSO4?
119. —колько граммов цинка и серной кислоты вступили в реакцию, если при этом выделилось 5,6 л водорода (н.у.)?
120. —колько граммов щелочи NaOH потребуетс€ дл€ раство≠рени€ 4 молей Zn(OH)2?
121. —колько раствора ќЌ концентрации 0,5 моль/л (0,5 н) потребуетс€ дл€ полной нейтрализации раствора, содержащего 6,5 г H2SO4?
122. ќпределите мол€рность и нормальность 70%-й Ќ—1O4 с р = 1,7 г/мл. —колько этой кислоты потребуетс€ дл€ приготов≠лени€ 200 мл раствора концентрации 4 моль/л (4 н)?
123. —колько эквивалентов заключаетс€ в 1 моле N2, H2S, —O2, —а—O3, —r2O3, Ќ3–O4?
124. акова мол€рность раствора Ќ3–O4 концентрации 1,5 моль/л (или 1,5 н)?
125. ƒл€ полного окислени€ 2,25 г металла потребовалось 1400 мл кислорода (н.у.). ќпределите эквивалентную массу металла и оксида.
126. —мешали растворы, содержащие по 1 г Ќ—l и ќЌ. ака€ будет среда: кисла€, щелочна€ или нейтральна€?
127. ¬ычислите, сколько граммов ¬а(ќЌ)2 потребуетс€ дл€ полной нейтрализации 100 мл раствора H2SO4 концентрации 1 моль/л (1 н).
128. ќпределите мол€рность и мол€рную концентрацию эквивалента (нормальность) 13,5%-й Ќ—1 с р = 1,065 г/мл. —колько этой кислоты потребуетс€ дл€ приготовлени€ 250 мл раствора концентрации 0,5 моль/л (0,5 н)?
129. ѕри сжигании 3,04 г металла было получено 5,04 г оксида. ќпределите атомную массу металла, зна€, что его валентность равна двум, и составьте формулу оксида.
130. акой объем раствора Ќ—1 концентрации 0,4 моль/л (0,4 н) следует прибавить к раствору AgNO3 дл€ получени€ 0,2867 г AgCl?
131. ќпределите титр “, эквивалентность N, мол€рность C, мол€льность раствора C м
| «адача | растворенное вещество | ω, % | плотность ρ, г/ см3 |
| 131 а | KOH | 1,27 | |
| 131 б | H2SO4 | 1,04 | |
| 131в | HNO3 | 1,316 | |
| 131 г | NaOH | 1,109 | |
| 131 д | A1—13 | 1,09 | |
| 131 е | BaCl2 | 1,203 | |
| 131 ж | CuSO4 | 1,107 | |
| 131 з | HCl | 1,100 | |
| 131 и | KOH | 1,092 | |
| 131 к | H2SO4 | 1,105 | |
| 131л | H3PO4 | 1,254 | |
| 131 м | HgCl2 | 1,041 | |
| 131 н | AgNO3 | 1,194 | |
| 131 о | HClO4 | 1,128 |
132. акой объем, мл, раствора јисх Vисх потребуетс€ дл€ приготовлени€ заданного объема Vзд раствора ¬зд заданной концентрации?
| «адача | ќбъем заданного раствора Vзд, мл | заданный раствор ¬зд | »сходный раствор јисх | ||
| ρзд, г/ мм | — вщества | ρисх, г/ мм | — вщества | ||
| 133а | 1,0051 | 1% H2SO4 | 1,0385 | 6 % H2SO4 | |
| 133 б | 1,0051 | 0, 1 ћ H2SO4 | 1,0385 | 6 % H2SO4 | |
| 133 в | 1,0051 | 0, 1 ћ H2SO4 | 1,0250 | 4% H2SO4 | |
| 133 г | 1,0032 | 0, 1 N HCl | 1,0132 | 3% HCl | |
| 133 д | 1,0032 | 1% HCl | 1,0230 | 5% HCl | |
| 133 е | 1,0032 | 0, 4 N HCl | 1,0230 | 5% HCl |






