1. Концентрация ионов водорода в растворе составляет 10-3 моль/л. Рассчитать значения pH, pOH и [ОН-] в данном растворе. Определить среду раствора.
Примечание. Для вычислений используются соотношения: lg10 a = a; 10lg a = а.
Решение.
1) 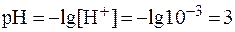 ;
;
2) 
3) 
Среда раствора с pH = 3 является кислой, так как pH < 7.
2. Вычислить рН раствора соляной кислоты с молярной концентрацией 0,002 моль/л.
Решение.
Так как в разбавленном растворе НС1  » 1, а в растворе одноосновной кислоты C(к-ты) = C(
» 1, а в растворе одноосновной кислоты C(к-ты) = C( к-ты), то можем записать:
к-ты), то можем записать:
1)  ,
,
2) 
3. К 10 мл раствора уксусной кислоты с C( СН3СООН) = 0,01 моль/л добавили 90 мл воды. Найти разность значений pН раствора до и после разбавления, если
СН3СООН) = 0,01 моль/л добавили 90 мл воды. Найти разность значений pН раствора до и после разбавления, если  (СН3СООН) = 1,85×10-5.
(СН3СООН) = 1,85×10-5.
Решение.
1) В исходном растворе слабой одноосновной кислоты СН3СООН:

Следовательно:
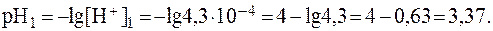
2) Добавление к 10 мл раствора кислоты 90 мл воды соответ-ствует 10-кратному разбавлению раствора. Поэтому:

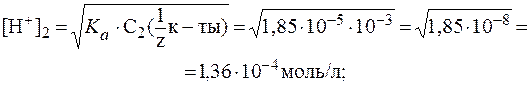

Таким образом:

4. Найти значение рН раствора гидроксида кальция с молярной концентрацией эквивалента 0,002 моль/л, если  = 95%.
= 95%.
Решение.
В растворах сильных оснований:
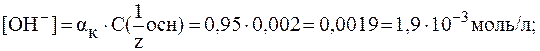
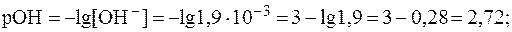
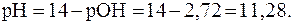
5. рН раствора серной кислоты с молярной концентрацией 0,001 моль/л равен 2,72. Найти  .
.
Решение.
В растворе сильной кислоты:
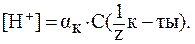
Кислота двухосновная, следовательно, сначала необходимо определить молярную концентрацию эквивалента H2SO4 в растворе:
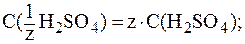


Зная величину pH раствора, можно рассчитать [H+]:

Отсюда:

6. Рассчитать рН раствора NaOH, если известно, что в 200 мл этого раствора содержится 0,0004 г NaOH ( » 1).
» 1).
Решение.
В разбавленном растворе сильного основания:

Рассчитаем C(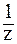 NaOH):
NaOH):



7. Вычислить число ионов гидроксида, содержащихся в 5 мл раствора, водородный показатель которого равен 3.
Решение.
1) 
2) 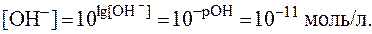
3) 
Для вычисления числа ионов используется соотношение, связывающее число структурных единиц (атомов, ионов, молекул) вещества - N(x), количество этих структурных единиц - n(x) и постоянную Авогадро NА, равную 6,02·1023 моль-1:
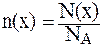 .
.
Отсюда:

8. Рассчитать массу основания С5H5N·Н2О в 150 мл раствора, водородный показатель которого равен 10, если  (С5H5N·Н2О) = 5,2.
(С5H5N·Н2О) = 5,2.
Решение.
Массу основания в растворе можно вычислить, зная молярную концентрацию раствора. Так как С5H5N·Н2О - однокислотное основание, то из соотношения 14′:
рС(осн) = 2·рОН -  .
.
Величину рОН найдем из соотношения:
рОН = 14 - рН = 14 - 10 = 4.
Таким образом:
рС(С5H5N·Н2О) = 2·4 - 5,2 = 2,8;
С(С5H5N·Н2О) = 10-рС = 10-2,8 = 1,58·10-3 моль/л;
m(С5H5N·Н2О) = С(С5H5N·Н2О)·М(С5H5N·Н2О)·Vр-ра =
= 1,58·10-3·97·0,15 = 0,023 г.
9. Вычислить молярную концентрацию гидроксида калия в растворе, водородный показатель которого равен 12, если  = 90 %.
= 90 %.
Решение.
Гидроксид калия является однокислотным основанием, поэтому согласно соотношениям (8), (10) и (13):

10. Вычислить рН раствора азотной кислоты с C(HNO3) = 0,01 моль/л (расчет вести через активность ионов Н+).
Решение.

Для определения коэффициента активности  сначала следует вычислить ионную силу раствора I:
сначала следует вычислить ионную силу раствора I:




Величину  , отвечающую I = 0,01, можно рассчитать по формуле:
, отвечающую I = 0,01, можно рассчитать по формуле:

Отсюда:


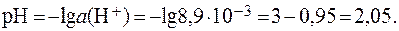
Если принять  = 1, то:
= 1, то:


Для точных расчетов сотые доли имеют значение.
11. Рассчитать рН раствора, в 100 мл которого находится 0,1 г гидроксида натрия и 0,174 г сульфата калия.
Решение.
Молярные концентрации электролитов в растворе составляют:
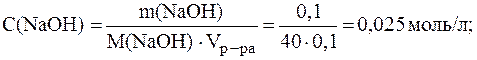

Ионная сила раствора, содержащего ионы Na+, K+, ОН- и SO42-, равна:

Коэффициент активности гидроксид-ионов и их активность соответственно равны:

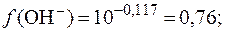

Из соотношения (3) находим активность ионов водорода:

Таким образом:

12. Найти число недиссоциированных молекул кислоты в 500 мл раствора HF, если 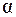 = 10 %, pH = 2,5,
= 10 %, pH = 2,5,  (НF) = 7,2×10-4.
(НF) = 7,2×10-4.
Решение.
В растворе слабой одноосновной кислоты молярную концентрацию кислоты можно рассчитать по формуле (12):
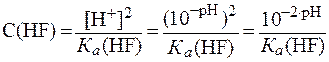 .
.
Общее количество кислоты (n0) в заданном объеме раствора равно:
 .
.
Количество недиссоциированной кислоты (n) найдем по формуле:
 .
.
Число недиссоциированных молекул кислоты равно:

Вопросы для самоконтроля
1. Какие ионы образуются при диссоциации воды? Составьте выражение для константы диссоциации воды.
2. Что называется ионным произведением воды? Каково численное значение 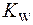 при 20-250С?
при 20-250С?
3. Чем может быть вызвано изменение величины ионного произведения воды?
4. Изменится ли ионное произведение воды при добавлении к ней кислоты, щелочи или соли?
5. Являются ли концентрации ионов Н+ и ОН- в водных растворах сопряженными величинами?
6. Может ли в водном растворе кислоты (щелочи) концентрация ионов Н+ или ОН- быть равной нулю?
7. Что понимают под терминами кислая, нейтральная, щелочная среда?
8. Что такое активная, потенциальная и общая кислотность в растворах кислот? Что называют активной реакцией среды?
9. Как определяют водородный и гидроксидный показатели? Какова взаимосвязь рН и рОН?
10. Что представляет собой шкала значений рН? Каковы значения рН в нейтральной, кислой и щелочной средах?
11. Имеет ли значение постоянство активной реакции среды в жизнедеятельности человека? Каковы значения рН важнейших биологических жидкостей (кровь, желудочный сок, моча, пот, слюна)?
12. Что такое ацидоз? Что такое алкалоз?
13. Какой вид имеют формулы для расчета активной кислотности в растворах сильных и слабых кислот и оснований?
14. Может ли присутствие NaCl оказать влияние на величину pH раствора соляной кислоты?
15. Могут ли величины рН и рОН принимать отрицательные значения?
Варианты задач для самостоятельного решения
Вариант №1
1. Определить [Н+] и [ОН-], если рН раствора равен 4.
2. Вычислить гидроксидный показатель желудочного сока, если известно, что 100 мл его содержит 0,365 г соляной кислоты ( » 1) и 0,585 г хлорида натрия.
» 1) и 0,585 г хлорида натрия.
3. Рассчитать массу основания NH3×Н2О в 500 мл раствора, водородный показатель которого равен 10,5, если 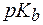 (NH3×Н2О) = 4,74.
(NH3×Н2О) = 4,74.
Вариант №2
1. Вычислить число ионов Н+, находящихся в 25 мл раствора, если рОН = 14.
2. Рассчитать рН раствора гидроксида кальция с C(Са(ОH)2) = 0,02 моль/л, если  = 90%.
= 90%.
3. Найти массу уксусной кислоты в 100 мл раствора, гидроксидный показатель которого равен 10, если  (СН3СООН) = 1,8×10-5.
(СН3СООН) = 1,8×10-5.
Вариант №3
1. Определить рН раствора и [Н+] в растворе, если [ОН-] = 3×10-5 моль/л. Указать характер среды.
2. Вычислить массу гидроксида бария в 250 мл раствора, водородный показатель которого равен 13, если  » 1.
» 1.
3. Рассчитать рН раствора бензойной кислоты с молярной концентрацией 0,01 моль/л, если  (С6Н5СООН) = 4,2. Во сколько раз изменится рН раствора при разбавлении его водой в 5 раз?
(С6Н5СООН) = 4,2. Во сколько раз изменится рН раствора при разбавлении его водой в 5 раз?
Вариант №4
1. Вычислить рН раствора и [Н+] в растворе, если 40 мл раствора содержат 12,04·1020 ионов гидроксида. Указать характер среды.
2. Рассчитать рОН раствора синильной кислоты с молярной концентрацией эквивалента 0,01 моль/л, если  (HCN) = 8×10-10.
(HCN) = 8×10-10.
3. Найти рН раствора, в 200 мл которого растворено 0,63 г азотной кислоты ( = 1) и 0,261 г нитрата бария.
= 1) и 0,261 г нитрата бария.
Вариант №5
1. Определить [Н+] и [ОН-] в растворе, рОН которого равен 5.
2. Вычислить молярную концентрацию гидроксида натрия в растворе, водородный показатель которого равен 13,5, если  = 75%.
= 75%.
3. Рассчитать величину рН раствора, в 250 мл которого находится 0,46 г муравьиной кислоты, если  (HCООН) = 2,2×10-4. Во сколько раз изменится рН раствора при разбавлении его водой в 3 раза?
(HCООН) = 2,2×10-4. Во сколько раз изменится рН раствора при разбавлении его водой в 3 раза?
Вариант №6
1. Рассчитать число ионов ОН-, находящихся в 2 мл раствора, если рН = 10.
2. К 100 мл раствора хлорной кислоты с C(НС1О4) = 0,1 моль/л ( » 1) прибавили 100 мл Н2О. Найти рОН раствора до и после разбавления.
» 1) прибавили 100 мл Н2О. Найти рОН раствора до и после разбавления.
3. Вычислить массу основания СН3NH2×Н2О в 650 мл раствора, водородный показатель которого равен 11,5, если  (СН3NH2×Н2О) = 4,4·10−4.
(СН3NH2×Н2О) = 4,4·10−4.
Вариант №7
1. Вычислить рОН раствора и [ОН-] в растворе, если [Н+] = 5×10-8 моль/л.
2. Рассчитать молярную концентрацию основания С2Н5NH2·H2O в растворе, водородный показатель которого равен 11,7, если  (С2Н5NH2×Н2О) = 3,25.
(С2Н5NH2×Н2О) = 3,25.
3. Найти рН раствора гидроксида калия, если известно, что 100 мл этого раствора содержит 0,0056 г КОН. ( » 1). Во сколько раз изменится рН раствора при разбавлении его водой в 2 раза?
» 1). Во сколько раз изменится рН раствора при разбавлении его водой в 2 раза?
Вариант №8
1. Определить [Н+] и [ОН-] в растворе, pH которого равен 6.
2. Вычислить рН раствора, в 500 мл которого находится 0,05 г LiOH. Диссоциацию щелочи считать полной. Во сколько раз изменится рН раствора при разбавлении его водой в 10 раз?
3. Найти массу азотистой кислоты, содержащейся в 800 мл раствора, гидроксидный показатель которого равен 11,4, если  (HNO2) = 4×10-4.
(HNO2) = 4×10-4.
Вариант №9
1. Определить рОН раствора, если 1250 мл раствора содержат 3,01·1023 ионов водорода.
2. Найти рН раствора фтороводородной кислоты, если в 10 мл этого раствора растворено 0,004 г HF, а  (HF) = 3,18. Во сколько раз изменится рН раствора при разбавлении его водой в 3 раза?
(HF) = 3,18. Во сколько раз изменится рН раствора при разбавлении его водой в 3 раза?
3. Вычислить массу гидроксида стронция в 300 мл раствора, водородный показатель которого равен 12,8, если кажущаяся степень диссоциации гидроксида равна 95%.
Вариант №10
1. Вычислить рН раствора и [Н+] в растворе, если [ОН-] = 4×10-4 моль/л. Определить характер среды.
2. Рассчитать массу соляной кислоты, содержащейся в 1,5 мл желудочного сока, водородный показатель которого равен 1,8, если  » 1.
» 1.
3. Определить водородный показатель раствора диметиламина с C((СН3)2NH×Н2О) = 0,01 моль/л, если  ((СН3)2NH×Н2О) = 6,1×10-4. Во сколько раз изменится рН раствора при разбавлении его водой в 15 раз?
((СН3)2NH×Н2О) = 6,1×10-4. Во сколько раз изменится рН раствора при разбавлении его водой в 15 раз?
Вариант №11
1. Определить [Н+] и [ОН-], если рН раствора равен 1.
2. Вычислить значение рН раствора гидроксида аммония, в 450 мл которого содержится 0,07 г основания, если  (NH3×Н2О) = 1,85·10−5.
(NH3×Н2О) = 1,85·10−5.
3. К 125 мл раствора серной кислоты с C(Н2SО4) = 0,03 моль/л ( » 91%) добавлено 125 мл воды. Найти рОН раствора до и после разбавления, если кажущаяся степень диссоциации увеличивается до 95%.
» 91%) добавлено 125 мл воды. Найти рОН раствора до и после разбавления, если кажущаяся степень диссоциации увеличивается до 95%.
Вариант №12
1. Вычислить число ионов Н+, находящихся в 30 мл раствора, если рОН = 1.
2. Найти рН раствора гидроксида рубидия, если в 120 мл этого раствора содержится 0,204 г RbОН ( » 1) и 0,87 г K2SO4.
» 1) и 0,87 г K2SO4.
3. Определить массу пропионовой кислоты в 750 мл раствора, гидроксидный показатель которого равен 11,5, если  (С2Н5СООН) = 4,89. Во сколько раз изменится рН раствора при разбавлении его водой в 7 раз?
(С2Н5СООН) = 4,89. Во сколько раз изменится рН раствора при разбавлении его водой в 7 раз?
Вариант №13
1. Определить [Н+] и [ОН-] в растворе, рОН которого равен 4.
2. Найти рН раствора, в 400 мл которого растворено 0,256 г иодоводородной кислоты, если  » 98%.
» 98%.
3. Рассчитать массу основания С6Н5NH2×Н2О в 1500 мл раствора, водородный показатель которого равен 9, если  (С6Н5NH2×Н2О) = 3,8·10−10. Во сколько раз изменится рН раствора при разбавлении его водой в 8 раз?
(С6Н5NH2×Н2О) = 3,8·10−10. Во сколько раз изменится рН раствора при разбавлении его водой в 8 раз?
Вариант №14
1. Определить рОН раствора и [ОН-], если 4 мл раствора содержат 24,08·1019 ионов водорода. Указать характер среды.
2. Вычислить молярную концентрацию бензойной кислоты в растворе, гидроксидный показатель которого равен 10,6, если  (С6Н5СООН) = 6,3×10-5. Во сколько раз изменится рН раствора при разбавлении его водой в 5 раз?
(С6Н5СООН) = 6,3×10-5. Во сколько раз изменится рН раствора при разбавлении его водой в 5 раз?
3. Рассчитать рН раствора, содержащего гидроксид калия с С(КОН) = 0,005 моль/л и нитрат натрия с С(NаNO3) = 0,015 моль/л.
Вариант №15
1. Вычислить [Н+] и [ОН-] в растворе, рОН которого равен 6,5.
2. Определить массу уксусной кислоты в 350 мл раствора, гидроксидный показатель которого равен 10,4, если  (СН3СООН) = 4,75.
(СН3СООН) = 4,75.
3. К 25 мл раствора гидроксида кальция с C(Сa(OH)2) = 0,015 моль/л ( » 90%) добавлено 125 мл воды. Найти рН раствора до и после разбавления, если кажущаяся степень диссоциации увеличивается до 99%.
» 90%) добавлено 125 мл воды. Найти рН раствора до и после разбавления, если кажущаяся степень диссоциации увеличивается до 99%.
Вариант №16
1. Определить число ионов ОН-, находящихся в 20 мл биологической жидкости, водородный показатель которой равен 7,35.
2. Рассчитать величину рОН раствора, в 50 мл которого находится 0,027 г бромоводородной кислоты, если  » 1. Во сколько раз изменится рН раствора при разбавлении его водой в 3 раза?
» 1. Во сколько раз изменится рН раствора при разбавлении его водой в 3 раза?
3. Вычислить массу основания СН3NH2×Н2О в 300 мл раствора, водородный показатель которого равен 11, если  (СН3NH2×Н2О) = 3,36.
(СН3NH2×Н2О) = 3,36.
Вариант №17
1. Вычислить рН раствора и [Н+] в растворе, если [ОН-] = 2×10-4 моль/л. Определить характер среды.
2. Найти рН раствора, в 500 мл которого находится 0,005 моль гидроксида натрия и 0,01 моль хлорида кальция.
3. Рассчитать массу циановодородной кислоты, содержащейся в 400 мл раствора, гидроксидный показатель которого равен 11,4, если  (HСN) = 9,1.
(HСN) = 9,1.
Вариант №18
1. Определить соотношение [ОН-] в крови (рН = 7,4) и в спинномозговой жидкости (рН = 7,5).
2. Найти водородный показатель раствора азотной кислоты с C(НNО3) = 10-9 моль/л, если  » 1.
» 1.
3. Рассчитать массу основания С2Н5NH2·H2O в 1800 мл раствора, водородный показатель которого равен 11,2, если  (С2Н5NH2×Н2О) = 5,6·10−4. Во сколько раз изменится рН раствора при разбавлении его водой в 5 раз?
(С2Н5NH2×Н2О) = 5,6·10−4. Во сколько раз изменится рН раствора при разбавлении его водой в 5 раз?
Вариант №19
1. Определить рОН раствора, если 190 мл раствора содержат 15,05·1013 ионов водорода.
2. Вычислить рН раствора гидроксида бария, если известно, что 750 мл этого раствора содержит 0,513 г Ва(ОН)2. Во сколько раз изменится рН раствора при разбавлении его водой в 20 раз? В обоих случаях диссоциацию щелочи считать полной.
3. К 10 мл раствора муравьиной кислоты с C(НСООН) = 0,15 моль/л прибавили 40 мл Н2О. Найти рОН раствора до и после разбавления, если  (HCООН) = 3,66.
(HCООН) = 3,66.
Вариант №20
1. Определить гидроксидный показатель раствора гидроксида цезия с C(CsОН) = 10-9 моль/л, если  » 1.
» 1.
2. Рассчитать число недиссоциированных молекул слабой одноосновной кислоты в 800 мл раствора, если степень диссоциации кислоты составляет 2,5%, рН раствора равен 3,5, а  (к-ты) = 1,85×10-5.
(к-ты) = 1,85×10-5.
3. Вычислить водородный показатель раствора, в 300 мл которого содержится 0,384 г иодоводородной кислоты и 0,284 г сульфата натрия.
БЛОК ИНФОРМАЦИИ






