Самая сильная кислота, которая может существовать и оставаться устойчивой в среде растворителя - ион лиония (в случае воды - это H3O+), а самое сильное основание - ион лиата (для воды ОН-). Кислоты, проявляющие более сильные кислотные свойства, чем ион лиония, и основания, проявляющие более сильные основные свойства, чем ион лиата, называются сильными.
Для частицы H3O+ pKa = 0, сильными считаются кислоты, у которых pKa < 0. К ним относятся, например, HClO4, HI, HBr, HCl, H2SO4, HNO3. Экспериментально определить различия в силе кислотности у данных соединений практически невозможно. В водном растворе они все будут вести себя практически одинаково.
HClO4 + H2O ® H3O+ + ClO4-
Основания, проявляющие более сильные основные свойства, чем ион OH- (pKBH+ = 14), в водном растворе также уравниваются по силе. К таким основаниям относятся NH2-, O2-, CH3-, H- и др.
NH2- + H2O ® NH3 + OH-
Кислоты, являющиеся более слабыми, чем ион лиония, и основания, проявляющие более слабыми основными свойствами, чем ион лиата (если только они не являются слишком слабыми кислотами или основаниями), проявляют индивидуальные свойства. У кислот средней силы величина pKa находится в интервале примерно 0 - 4 (границы условны!).
HSO4- + H2O › SO42- + H3O+ (pKa =1,94)
У слабых кислот pKa > 4:
H2S + H2O › HS- + H3O+ (pKa = 6,99)
Основания, имеющие pKBH+ от 10 до 14, иногда называют основаниями средней силы, а основания, у которых pKBH+ < 10, - слабыми.
S2- + H2O › HS- + OH- (pKBH+ = 12,6)
C6H5NH2 + H2O › C6H5NH3+ + OH- (pKBH+ = 4,6)
Уравнивание по силе кислот более сильных, чем ион лиония, и оснований более сильных, чем ион лиата, а также очень слабых кислот и оснований называется нивелирующим действием растворителя.
Не слишком сильные и не слишком слабые кислоты и основания можно практически различить по силе. Растворитель оказывает на них дифференцирующее действие (рис. 4.1).

Рис. 4.1. Значения pH 0,1 М кислот в воде
Способность растворителя оказывать нивелирующее или дифференцирующее действие зависит от:
· кислотно-основных свойств растворителя;
· его склонности к автопротолизу.


В аммиаке невозможно отличить по силе не только хлорную и хлороводородную кислоты, но и хлорную и уксусную, т.к. NH4+ является значительно более слабой кислотой, чем H3O+. Таким образом, аммиак обладает ещё более сильным, чем вода, нивелирующим действием на кислоты.
Растворитель с сильными основными свойствами нивелирует силу кислот и дифференцирует силу оснований. Сильно кислотный растворитель, наоборот, дифференцирует силу кислот и нивелирует силу оснований.
Чем меньше величина константы автопротолиза растворителя, тем больше вероятность того, что он будет оказывать дифференцирующее действие на силу кислот и оснований (рис. 4.2).

Рис. 4.2. Константа автопротолиза растворителя и его дифференцирующее действие
4.5. Расчёт рН водных растворов различных протолитов
Водородным показателем (рН) называется отрицательный десятичный логарифм активности (или молярной концентрации) ионов водорода в растворе.
pH 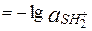 pH
pH 
Для водных растворов
pH  pH
pH 
Для концентрированных растворов величины рН, рассчитанные через активность и молярную концентрацию, отличаются, поэтому для них иногда даже используют разные обозначения, соответственно, paH и pcH. Обозначение рН используется для экспериментально определяемой величины, которая в точности не соответствует ни paH ни pcH, но ближе к первой, чем ко второй.
Для характеристики кислотности и щёлочности водных растворов используется интервал рН от 0 до 14. В более кислых и более щелочных растворах понятие рН теряет смысл, так как активность и концентрация H3O+ обычно значительно отличаются, причём еще и по-разному у различных веществ.
Растворы сильных кислот или сильных оснований
В водном растворе сильной кислоты имеются следующие протолитические равновесия:
HA + H2O ® H3O+ + A- H2O + H2O › H3O+ + OH-
Если CHA > 10-6 моль/л, то ионами H3O+, образующимися при автопротолизе воды, можно пренебречь. Тогда
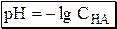
Если CHA < 10-6 моль/л, то необходимо учесть и те протоны, которые образовались при автопротолизе.

 ,
,  ,
, 
 или
или 
В данном случае физический смысл имеет только один из корней полученного квадратного уравнения, так как концентрация не может быть отрицательной.


Аналогичные формулы можно получить и для сильных оснований.


Пример 4.1. Рассчитать рН 0,01 М HCl и 0,01 М NaOH, а также 1,0×10-8 М HCl.
1) pH = -lg0,01 = 2,0 2) pH = 14,0 + lg0,01 = 12,0
3) pH  = 6,98
= 6,98
Точный расчёт рН для последнего случая имеет чисто теоретический интерес, поскольку наличие в растворе даже незначительных количеств примесей (например, растворённого CO2) приведёт к заметно большему изменению рН, чем присутствие в растворе такого ничтожного количества HCl.
Растворы слабых кислот или слабых оснований
В водном растворе слабой кислоты, наряду с автопротолизом воды, имеется следующее протолитическое равновесие
HA + H2O › H3O+ + A-

Если кислота достаточно слабая (степень протолиза менее 5%), то можно принять, что [HA]» CHA. Если не учитывать автопротолиз воды,  .
.



Если степень протолиза превышает 5%




Степень протолиза кислоты зависит от её константы кислотности и концентрации в растворе:

 или pKa + lgCHA ³ 2,6
или pKa + lgCHA ³ 2,6
Для 0,1 М CH3COOH pKa + lgCHA = 4,75 – 1 = 3,75 (a < 5%), для 0,1 М H3PO4 pKa + lgCHA = 2,15 – 1 = 1,15 (a > 5%)
Если кислота очень слабая или концентрация её слишком мала (СHAKa < 10-12 или pKa – lgCHA < 12), то уже нельзя считать, что 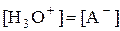 , поскольку необходимо учесть автопротолиз воды.
, поскольку необходимо учесть автопротолиз воды.




В водном растворе слабого основания имеется следующее равновесие, описываемое константой основности
B + H2O › BH+ + OH-
Для вывода формулы для расчёта рН раствора слабого основания рассмотрим взаимодействие кислоты, сопряжённой с рассматриваемым основанием, с водой. Такое равновесие описывается KBH+
BH+ + H2O › B + H3O+

Приняв, что [B]» CB, и так как [BH+] = [OH-] = 



Если нельзя принять, что [B] ¹ CB, то [B] = CB – [OH-]



Пример 4.2 Рассчитать рН 0,10 М CH3COOH (pKa = 4,76), 0,10 М CCl3COOH (pKa = 0,70, Ka = 0,20), 0,10 М NH4Cl (pKa(NH4+)= 9,24) 0,10 М NH3 и 0,10 М CH3COONa
1) рН 
2) pKa + lgCHA = 0,7 – 1 = -0,7 (степень протолиза больше 5%)
рН  = 1,14
= 1,14
3) рН  4) рН
4) рН  = 11,1
= 11,1
5) рН  = 8,9
= 8,9
Смеси кислот или оснований и многопротонные протолиты
Пусть в растворе присутствуют две кислоты HA1 и HA2, имеющие константы кислотности, соответственно, Ka1 и Ka2.



Если степень протолиза кислот меньше 5%, то их равновесные концентрации можно заменить общими. Кроме того, если Ka[HA] >> KW, то автопротолиз воды можно не учитывать.
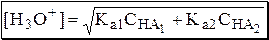
Для n слабых кислот

Если произведение KaC для двух кислот значительно отличаются, то при расчёте рН влиянием той из них, для которой это произведение значительно меньше, можно пренебречь.
Рассмотрим случай, когда одна из кислот, например, HA1 является сильной, а вторая - слабой.


С учётом того, что для слабой кислоты 

Если в растворе присутствуют два слабых основания, то уравнение электронейтральности (без учёта автопротолиза) имеет следующий вид


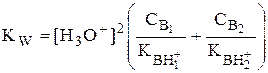

Полученные формулы применимы и для многоосновных кислот и многокислотных оснований. Например, многоосновную кислоту можно рассматривать как смесь кислот (например, H2A и HA-). Так как обычно Ka1 >> Ka2 и [H2A] во много раз превышает [HA-], то расчёт проводят по тем же формулам, что и для одноосновных кислот.
Пример 4.3. Рассчитать рН: 1) раствора, содержащего 0,10 моль/л CH3COOH (Ka = 1,75×10-5) и 0,10 моль/л HCOOH(Ka = 1,8×10-4);2) 0,10 М аскорбиновой кислоты (pKa1 = 4,04, pKa2 = 11,34); 3) 0,1 М Na2CO3 (для угольной кислоты pKa1 = 6,35, pKa2 = 10,32)
1) 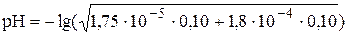 = 2,35
= 2,35
2) 
3)  = 11,7
= 11,7
Растворы амфолитов
Рассмотрим поведение амфолита HA- (например, HCO3-) в водном растворе.
HA- + H2O › H3O+ + A2- HA- + H3O+ › H2A + H2O
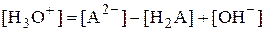
 и
и  , следовательно:
, следовательно:


Если  , то
, то

Если [HA-]» CHA- и [HA-] >> Ka1, то


Такие же формулы используются и для амфолитов типа BH+A-
Пример 4.4. Рассчитать рН 0,10 М NaHCO3 и 0,10 М HCOONH4
1) 
2) 
4.6. Расчёт состава равновесных смесей протолитов при заданном значении рН
Рассмотрим двухосновную кислоту H2A.



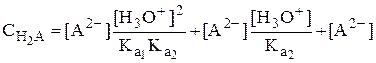 =
=  =
=
= 
Молярные доли частиц будут равны:


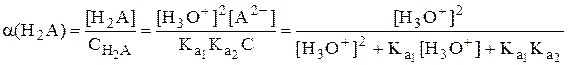
В общем виде формула для расчёта молярной доли частицы  имеет следующий вид
имеет следующий вид

Для одноосновной кислоты


С учётом того, что  и
и  :
:


Из данных формул следует, что при рН = pKa a(HA) = a(A-) = 0,5. Если рН превышает рKa на 1, то молярная доля А- в 10 раз больше, чем HA, если на 2 - то в 100 раз, на 3 - в 1000 и т.д. При уменьшении рН аналогичным образом увеличивается a(HA) (рис. 4.3).
Если значения Ka для некоторой многоосновной кислоты отличаются друг от друга на 4 и более порядка, то можно считать, что при любом значении рН в равновесной смеси будут присутствовать только два вида частиц, а концентрация остальных пренебрежимо мала. Например, если необходимо рассчитать молярную долю молекул H3PO4 (pKa1 = 2,15; pKa2 = 7,21) при рН 3, то можно принять, что в равновесной смеси присутствуют только частицы H3PO4 и H2PO4-.Рассчёт можно провести по той же формуле, что и для одноосновной кислоты.
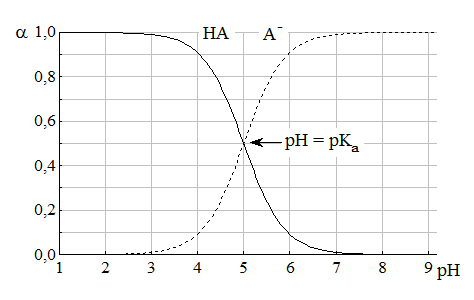
Рис. 4.3. Распределительная диаграмма для слабой кислоты (pKa = 5)
Пример 4.5. Рассчитать [NH3] в растворе с общей концентрацией аммиака 0,10 моль/л при рН 7,0.
 6,3×10-3
6,3×10-3
 М
М






