При решении задач № 51–94, 97 необходимо провести вычисления в следующей последовательности:
– написать уравнение реакции взаимодействия веществ, при необходимости по ступеням;
– перевести концентрации всех веществ в молярные;
– рассчитать количество молей всех веществ;
– на основании полученных данных определить состав раствора после протекания реакции и выбрать уравнение для расчета рН;
– рассчитать объем раствора после смешения;
– рассчитать концентрации веществ в образовавшемся после смешения растворе;
– вычислить рН раствора.
Примеры 8–9 помогут Вам при решении задач № 51–94, 97.
Пример 8. Вычислить рН раствора, полученного при сливании:
а) 20,0 мл 0,12 М раствора NaCN и 15,0 мл 0,09 М раствора HCl.
Решение. Запишем уравнение реакции
NaCN + HCl = HCN + NaCl
С целью выяснения состава раствора, образовавшегося после сливания, рассчитаем количества веществ в исходных растворах:
n0(NaCN) = 20,0 ∙ 10–3 ∙ 0,12 = 2,4 ∙ 10–3 моль;
n0(HCl) = 15,0 ∙ 10–3 ∙ 0,09 = 1,35 ∙ 10–3 моль.
Так как n0(NaCN) > n0(HCl), то NaCN находится в избытке, следовательно, в образовавшемся после сливания растворе будут находиться NaCN и HCN в следующих количествах:
n1(NaCN) = n0(NaCN) – n0(HCl) = 2,4 ∙ 10–3 – 1,35 ∙ 10–3 =
= 1,05 ∙ 10–3 моль.
n1(HCN) = n0(HCl) = 1,35 ∙ 10–3 моль.
Объем раствора (V) составит 20 + 15 = 35 мл.
Рассчитаем концентрации веществ в растворе:
С(NaCN) = 1,05 ∙ 10–3/ 35 ∙ 10–3 = 0,03 моль/л.
С(HCN) = 1,35 ∙ 10–3/ 35 ∙ 10–3 = 0,039 моль/л.
Исходя из состава раствора, выбираем формулу (7) для расчета рН буферных растворов:
pH = 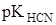 – lg[С(HCN) / С(NaCN)] = 9,3 – lg(0,039/0,03) = 9,18;
– lg[С(HCN) / С(NaCN)] = 9,3 – lg(0,039/0,03) = 9,18;
б) 11,25 мл 0,12 моль/л раствора NaCN и 15,0 мл 0,09 моль/л раствора HCl.
Решение.
n(NaCN) = 11,25 ∙ 10–3 ∙ 0,12 = 1,35 ∙ 10–3 моль.
n(HCl) = 15,0 ∙ 10–3 ∙ 0,09 = 1,35 ∙ 10–3 моль.
Так как n(NaCN) = n(HCl), то в образовавшемся после сливания растворе будет находиться только 1,35 ∙ 10–3 моль HCN.
Объем раствора (V) составит 11,25 + 15,0 = 26,25 мл.
Рассчитаем концентрацию HCN в растворе:
С(HCN) = 1,35 ∙ 10–3/ 26,25 ∙ 10–3 = 0,051 моль/л.
Исходя из состава раствора выбираем формулу (2) для расчета рН слабых кислот:
pH = 1/2 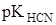 – 1/2 lg[С(HCN)] = 1/2 ∙ 9,3 – 1/2 ∙ lg(0,051) = 5,3;
– 1/2 lg[С(HCN)] = 1/2 ∙ 9,3 – 1/2 ∙ lg(0,051) = 5,3;
в) 20,0 мл 0,12 моль/л раствора NaCN и 35,0 мл 0,09 моль/л раствора HCl.
Решение.
n0(NaCN) = 20,0 ∙ 10–3∙ 0,12 = 2,4 ∙ 10–3 моль.
n0(HCl) = 35,0 ∙ 10–3 ∙ 0,09 = 3,15 ∙ 10–3 моль.
Так как n0(HCl) > n0(NaCN), т. е. в избытке HCl, то в образовавшемся после сливания растворе будут находиться HCl и HCN в следующих количествах:
n1(HCl) = 35,0 ∙ 10–3 ∙ 0,09 – 20,0 ∙ 10–3 ∙ 0,12 = 0,75 ∙ 10–3 моль;
n1(HCN) = 20,0 ∙ 10–3 ∙ 0,12 = 2,4 ∙ 10–3 моль.
Поскольку сильная кислота HCl подавляет диссоциацию слабой кислоты HCN, то рН определяется только ее концентрацией.
Объем раствора (V) составит 20,0 + 35,0 = 55,0 мл.
Рассчитаем концентрацию HCl в растворе:
С(HCl) = 0,75 ∙ 10–3/ 55,0 ∙ 10–3 = 0,014 моль/л.
Исходя из состава раствора, выбираем формулу (1) для расчета рН сильных кислот:
pH = – lg[С(HCl)] = – lg(0,014) = 1,87.
Пример 9. Вычислить рН раствора, полученного при сливании 10,0 мл 0,1 моль/л раствора Na2HAsO4 и 16,0 мл 0,1 моль/л раствора HCl.
Решение. После сливания растворов могут протекать следующие реакции:
Na2HAsO4 + HCl = NaH2AsO4 + NaCl (10)
NaH2AsO4 + HCl = H3AsO4 + NaCl (11)
Рассчитаем количества вещества в исходных растворах:
n(Na2HAsO4) = 10,0 ∙ 10–3 ∙ 0,1 = 1,0 ∙ 10–3 моль.
n(HCl) = 16,0 ∙ 10–3 ∙ 0,1 = 1,6 ∙ 10–3 моль.
Так как Na2HAsO4 взят в недостатке, то всё количество его прореагирует с HCl согласно уравнению (10), и после протекания реакции (10) в растворе останется 1,0 ∙ 10–3 моля NaH2AsO4 и (1,6 – 1,0) ∙ 10–3 =
= 0,6 ∙ 10– 3 моль HCl. Аналогично после реакции (11) в растворе будут находиться H3AsO4 и NaH2AsO4 в следующих количествах:
n(NaH2AsO4) = 1,0 ∙ 10–3 – 0,6 ∙ 10–3 = 0,4 ∙ 10–3 моль;
n(H3AsO4) = 0,6 ∙ 10–3 моль.
Объем раствора после смешения составит 10 + 16 = 26 мл, или 26 ∙ 10–3 л. Рассчитаем концентрации компонентов в растворе:
С(NaH2AsO4) = 0,4 ∙ 10–3/ 26 ∙ 10–3 = 0,015 моль/л;
С(H3AsO4) = 0,6 ∙ 10–3/ 26 ∙ 10–3 = 0,023 моль/л.
Исходя из состава раствора, выбираем формулу (7) для расчета рН буферных растворов:
pH =  – lg(С(H3AsO4) / С(NaH2AsO4)) =
– lg(С(H3AsO4) / С(NaH2AsO4)) =
= 2,22 – lg(0,023/0,015) = 2,03.
Решение задач № 95, 96 и 98–100 проводится непосредственно по уравнениям для расчета рН.






