ѕриклад 1.
ќбчислити  х≥м≥чноњ реакц≥њ CS2(р) + 3ќ2(г) = CO2(г) + 2Sќ2(г), при стандартних умовах ≥ зробити висновок про тепловий ефект реакц≥њ.
х≥м≥чноњ реакц≥њ CS2(р) + 3ќ2(г) = CO2(г) + 2Sќ2(г), при стандартних умовах ≥ зробити висновок про тепловий ефект реакц≥њ.
–озвТ€занн€.
«а насл≥дком ≥з закону √еса тепловий ефект реакц≥њ (DЌ0х.р.) дор≥внюЇ сум≥ теплот утворенн€ продукт≥в реакц≥њ, зменшен≥й на суму теплот утворенн€ вих≥дних речовин з урахуванн€м коеф≥ц≥Їнт≥в перед формулами цих речовин у р≥вн€нн≥ реакц≥њ:
DЌ0 х.р. = SDЌ0 прод. Ц SDЌ0 вих.реч.
« таблиц≥ 2 (додаток), де м≥ститьс€термодинам≥чн≥ константи речовин, знаходимо: DЌ0 (CS2) = 115,30 кƒж/моль, DЌ0 (CO2) = Ц 393,5 кƒж/моль, DЌ0 (Sќ2) = Ц 296,9 кƒж/моль.
” даному випадку:
DЌ0 х.р. = (DЌ0 298(CO2) + 2 DЌ0 298(Sќ2)) Ц (DЌ0 298(CS2) + 3 DЌ0 298(O2)); оск≥льки DЌ0 298 простих речовин дор≥внюють 0, то
DЌ 0х.р. = (DЌ0 298(CO2) + 2 DЌ0 298(Sќ2)) Ц DЌ0 298(CS2)= Ц393,5 кƒж/моль +2 × (Ц296,9 кƒж/моль) - 115,30 кƒж/моль = 85 кƒж/моль
якщо DЌ0 х.р.> 0, то реакц≥€ ендотерм≥чна ≥ супроводжуЇтьс€ поглинанн€м тепла, €кщо DЌ0 х.р.< 0, то реакц≥€ Ї екзотерм≥чною та супроводжуЇтьс€ вид≥ленн€м теплоти.
” нашому випадку реакц≥€ Ї ендотерм≥чною.
ѕриклад 2. ќбчисл≥ть зм≥ну енерг≥њ √≥бса у х≥м≥чн≥й реакц≥њ
4NH3(г) + 5O2(г) = 6H2O(г) + 4Nќ(г)
≤ зроб≥ть висновки про можлив≥сть мимов≥льного переб≥гу даноњ реакц≥њ за стандартних умов.
–озвТ€занн€. ≈нерг≥€ √≥бса (G) Ц термодинам≥чна функц≥€ стану системи, тому зм≥ну енерг≥њ √≥бса (DGх.р.) можна розрахувати за насл≥дком ≥з закону √еса за стандартних умов
DG0х.р. = SDG0прод. Ц SDG0вих.реч.,
де DG0прод. Ц зм≥ни енерг≥й √≥бса продукт≥в реакц≥њ, DG0вих.реч. Ц зм≥ни енерг≥й √≥бса вих≥дних речовин.
—тандартн≥ зм≥ни енерг≥й √≥бса простих речовин прийн€то вважати р≥вними нулю. ƒл€ даноњ системи:
DG0298,х.р. = (6 DG0298 (Ќ2ќ) + 4 DG0298 (Nќ)) Ц (4 DG0298 (NH3) + 5 DG0298 (ќ2));
оск≥льки DG0298 (ќ2) = 0, то
DG0298,х.р. = (6 DG0298 (Ќ2ќ) + 4 DG0298 (Nќ)) Ц 4 DG0298 (NH3).
¬з€вши в таблиц≥ 2 потр≥бн≥ дан≥ DG0298, знаходимо:
DG0298,х.р. = 6 × (Ц228,8) + 4 × (86,89) Ц 4 × (Ц16,64) = Ц959,48 кƒж.
¬еличина DGх.р. Ї м≥рою х≥м≥чноњ спор≥дненост≥ реагуючих речовин. ћимов≥льно переб≥гають х≥м≥чн≥ процеси, дл€ €ких зм≥на енерг≥њ √≥бса менше нул€. якщо DGх.р. > 0, процес за даних умов термодинам≥чно неможливий, а €кщо DGх.р = 0, то система знаходитьс€ у термодинам≥чн≥й р≥вноваз≥. ” даному випадку DG0298,х.р. < 0, отже, цей процес термодинам≥чно можливий за стандартних умов.
«авданн€ дл€ самост≥йноњ роботи
«авданн€ 1.
ќбчислити  х≥м≥чноњ реакц≥њ при стандартних умовах ≥ зробити висновок про тепловий ефект реакц≥њ
х≥м≥чноњ реакц≥њ при стандартних умовах ≥ зробити висновок про тепловий ефект реакц≥њ
| є вар≥анту | «авданн€ |
H2O(p) + SO3(г)  H2SO4(p) H2SO4(p)
| |
CaCl2(p) + Na2CO3(p) 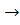 CaCO3(m) + NaCl(p) CaCO3(m) + NaCl(p)
| |
Na2SO4(p) + H2O(p)  H2SO4(p) + NaOH(p) H2SO4(p) + NaOH(p)
| |
Fe(OH)3(m) + HCl(p) 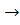 FeCl3(p) + H2O(p) FeCl3(p) + H2O(p)
| |
SnCl2(p) + 2FeCl3(p)  2FeCl2(p) + SnCl4(p) 2FeCl2(p) + SnCl4(p)
| |
8HI(г) + H2SO4(p) 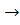 4I2(m) + H2S(г) + 4H2O(p) 4I2(m) + H2S(г) + 4H2O(p)
| |
NO2(г) + H2SO3(p)  H2SO4(p) + NO(г) H2SO4(p) + NO(г)
| |
Ca(OH)2(k) + CO2(г)  CaCO3(k) + H2O(p) CaCO3(k) + H2O(p)
| |
SO2(г) + H2O(p) + NO2(г)  H2SO4(p) + NO(г) H2SO4(p) + NO(г)
| |
Fe(k) + H2SO4(p)  FeSO4(p) + H2(г) FeSO4(p) + H2(г)
| |
CaCO3(k)  CaO(k) + CO2(г) CaO(k) + CO2(г)
| |
| Fe(k) + 4HNO3(p) = Fe(NO3)3(p) + NO(г) + 2H2O(p) | |
CaCO3(k) + 2HCl(p)  CaCl2(p) + H2O(p) + CO2(г) CaCl2(p) + H2O(p) + CO2(г)
| |
I2(m) + H2SO3(p) + H2O  H2SO4(p) + 2HI(г) H2SO4(p) + 2HI(г)
| |
Zn(m) + HCl(p)  ZnCl2(p) + H2(г) ZnCl2(p) + H2(г)
| |
C2H5OH(г)  C2H2(г) + H2O (n) C2H2(г) + H2O (n)
| |
H2S(г) + CO2(г)  H2O(n) + CS2(г) H2O(n) + CS2(г)
| |
CH4(г) + H2S(г) 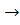 CS2(г) + H2(г) CS2(г) + H2(г)
| |
NO2(г) + H2O(p)  HNO3(p) + HNO2(p) HNO3(p) + HNO2(p)
|
|
|
|






