ѕриклад 1. ” 250 г води розчинено 50 г кристалог≥драту FeSO4 × 7Ќ2ќ. ќбчислити масову частку кристалог≥драту та безводного сульфату зал≥за (≤≤) у розчин≥.
–озвТ€занн€. ћаса отриманого розчину складаЇ 250 + 50 = 300 г. ћасову частку кристалог≥драту знаходимо за формулою

ќбчислюЇмо масу безводноњ сол≥ в 50 г кристалог≥драту. ћол€рна маса FeSO4 ×7Ќ2O дор≥внюЇ 278 г/моль, а мол€рна маса FeSO4 складаЇ 152 г/моль. ¬м≥ст FeSO4 у 50 г FeSO4 × 7Ќ2ќ знаходимо з пропорц≥њ
у 278 г FeSO4 × 7Ќ2ќ м≥ститьс€ 152 г FeSO4;
а в 50 г FeSO4 × 7Ќ2ќ Ц ’ г FeSO4;
’ = (50 × 152): 278 = 27,34 г.
«в≥дси масова частка безводноњ сол≥ в 300 г розчину дор≥внюЇ:
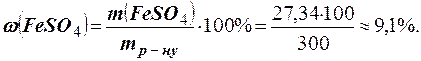
ѕриклад 2. « 200 г водного розчину з масовою часткою розчиненоњ речовини 50% при охолодженн≥ вид≥лилос€ 20 г ц≥Їњ речовини. ќбчислити масову частку речовини в розчин≥, що залишивс€.
–озвТ€занн€. ¬изначаЇмо масу речовини у вих≥дному розчин≥
 г.
г.
ћаса розчину п≥сл€ охолодженн€ дор≥внюЇ 200 Ц 20 = 180 г, а маса сол≥ становитиме: 100 Ц 20 = 80 г. “аким чином, обчислюЇмо масову частку сол≥ в розчин≥, що залишивс€: w = (80 × 100): 180 = 44,44%.
ѕриклад 3. ќбчислити мол€рну концентрац≥ю розчину ¬а—l2, у 40 мл €кого м≥ститьс€ 1,04 г розчиненоњ речовини. якою Ї його екв≥валентна мол€рна концентрац≥€?
–озвТ€занн€. ћол€рна маса ¬а—l2 становить 208 г/моль, а екв≥валентна маса ≈ (¬а—l2) = ћ (¬а—l2): 2 = 104 г/моль. ќбТЇм розчину Ц 0,04 л. ќбчислюЇмо мол€рну й екв≥валентну мол€рну концентрац≥њ ¬а—l2
 моль/л.
моль/л.
ѕ≥дставивши зам≥сть ћ (¬а—l2) ≈ (¬а—l2), маЇмо —eq (¬а—l2) = 0,25 моль/л.
ѕриклад 4. ќбчислити мол€рну концентрац≥ю розчину з масовою часткою Ќ—l 36,5%, густина €кого r = 1,18 г/см3.
–озвТ€занн€. ќбчислюЇмо масу 1 л (1000 см3) розчину
mр-ну = r × V = 1,18 × 1000 = 1180 г.
¬изначаЇмо масу розчиненоњ речовини в 1180 г розчину
 ;
;  г.
г.
¬изначаЇмо —ћ (Ќ—l) у розчин≥, знаючи, що ћ (Ќ—l) = 36,5 г/моль
 моль/л.
моль/л.
ѕриклад 5. який обТЇм розчину азотноњ кислоти з масовою часткою ЌNO3 w = 20% (r = 1,115 г/см3) ≥ €кий обТЇм води треба вз€ти дл€ приготуванн€ розчину ЌNO3 з масовою часткою w = 4% (r = 1,025 г/см3)?
–озвТ€занн€. «находимо масу заданого розчину (m2): m2 = r2 × V2 = 1,025 × × 2500 = 2562,5 г. ¬изначаЇмо масу ЌNO3 у цьому розчин≥
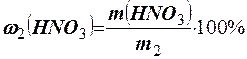 ;
;  г.
г.
«находимо масу вих≥дного розчину ЌNO3 (m1)
 ;
; 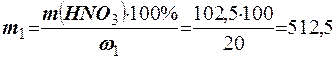 г.
г.
ƒал≥ визначаЇмо обТЇм вих≥дного розчину
V1 = m1 : r1 = 512,5: 1,115 = 459,6 см3.
Ќарешт≥ знаходимо масу води: m (Ќ2ќ) = m2 Ц m1 = 2562,5 Ц 512,5 = 2050 г.
ѕриклад 6. який обТЇм розчину з масовою часткою NaOH 36% (r2 = 1,39 г/см3) треба додати до 200 мл розчину з масовою часткою NaOH 11% (r1 = 1,12 г/см3), щоб одержати розчин ≥з масовою часткою 20%.

 –озвТ€занн€. «а правилом зм≥шуванн€ знаходимо сп≥вв≥дношенн€ мас вих≥дних розчин≥в: 36 9
–озвТ€занн€. «а правилом зм≥шуванн€ знаходимо сп≥вв≥дношенн€ мас вих≥дних розчин≥в: 36 9

 20
20
11 16
–озчину з масовою часткою NaOH 36% треба вз€ти 9 масових частин, а розчину з масовою часткою 11% Ц 16 масових частин.
«найдемо масу розчину з масовою часткою NaOH 11%:
|
|
|
m1 = r1 × V1 = 1,12 × 200 = 224 р.
«найдемо масу розчину з масовою часткою NaOH 36%:
16:9 = 224: m2; m2 = (224 × 9): 16 = 126 р.
¬изначимо обТЇм розчину з масовою часткою NaOH 36%:
V2 = m2 : r2 = 126: 1,39 = 90,65 см3.
ѕриклад 7. ƒо розчину ќЌ (r1 = 1,32 г/см3) обТЇмом 50 мл додали воду обТЇмом 150 мл. ќдержавс€ розчин ќЌ з масовою часткою 10%. ќбчисл≥ть масову частку (%) ќЌ у вих≥дному розчин≥.
–озвТ€занн€. «найдемо масу вих≥дного розчину (m1)
m1 = r1 × V1 = 1,32 × 50 = 66 г.
¬изначимо масу розведеного розчину (m2)
m2 = m1 + m (Ќ2ќ) = 66 + 150 = 216 г.
ќбчислимо масу OЌ в отриманому розчин≥
 г.
г.
ј масова частка OЌ у вих≥дному розчин≥:
 .
.
«авданн€ дл€ самост≥йноњ роботи
«авданн€ 1.
1. ƒо €кого обТЇму треба довести розчин при розчиненн≥ 13,3 г хлориду алюм≥н≥ю, щоб мол€рна концентрац≥€ одержаного розчину становила 0,2 моль/л?
2. ƒл€ приготуванн€ розчину з масовою часткою CuCl2 26% вз€то 800 г CuCl2 Ј 2Ќ2ќ. «найд≥ть масу одержаного розчину.
«авданн€ 2.
1. «м≥шали розчини азотноњ кислоти обТЇмом 5 мл ≥з масовою часткою HNO3 50% (ρ = 1,31 г/см3) та обТЇмом 50 мл ≥з масовою часткою HNO3 10% (ρ = 1,055 г/см3), та додали до них воду до 100 мл. якою Ї масова частка HNO3 в одержаному розчин≥ та €к≥ його мол€рна та екв≥валентна мол€рна концентрац≥њ?
2. ¬изначити обТЇм розчину з екв≥валентною мол€рною концентрац≥Їю 2 моль/л, €кий можна приготувати з 28,6 г Na2CO3 Ј 10Ќ2ќ.
«авданн€ 3
1. «найд≥ть масову частку н≥трату кал≥ю у розчин≥, у 500 мл €кого м≥ститьс€ 100 г KNO3 (ρ = 1,118 г/см3).
2. ќбчисл≥ть обТЇм розчину карбонату кал≥ю з масовою часткою 2—ќ3 16% (ρ = 1,14 г/см3), €кий потр≥бний дл€ приготуванн€ розчину 2—ќ3 обТЇмом 3 л з його Ceq ( 2—ќ3) = 0,5 моль/л.
«авданн€ 4.
1. —к≥льки хлориду кальц≥ю м≥ститьс€ у 100 мл розчину з масовою часткою —аCl2 30% (ρ = 1,282 г/см3)?
2. ƒо 100 мл розчину H2SO4 з масовою часткою 62% (ρ = 1,52 г/см3) додали 760 мл води. ќдержали розчин з густиною 1,52 г/см3. ќбчисл≥ть масову частку H2SO4 та Ceq одержаного розчину.
«авданн€ 5.
1. —к≥льки к≥лограм≥в розчину з масовою часткою CuSO4 4% треба додати до 2 кг розчину з масовою часткою 12%, щоб одержати розчин ≥з масовою часткою 10%?
2. який обТЇм розчину Na2SO4 з мол€рною концентрац≥Їю 0,2 моль/л треба вз€ти дл€ приготуванн€ 3 л розчину з Ceq = 0,16 моль/л?
«авданн€ 6.
1. —к≥льки грам≥в FeSO4 м≥ститьс€ у 5 л розчину з екв≥валентною мол€рною концентрац≥Їю 0,4 моль/л?
2. ƒо €кого обТЇму сл≥д розвести 7 л розчину HNO3 ω = 48% (ρ = 1,3 г/см3), щоб одержати розчин з мол€рною концентрац≥Їю 2 моль/л?
«авданн€ 7.
1. —к≥льки грам≥в AgNO3 м≥стить 20 мл розчину з мол€рною концентрац≥Їю 1 моль/л. якою Ї його екв≥валентна мол€рна концентрац≥€?
2. ќбчисл≥ть обТЇм розчину г≥дроксиду натр≥ю з масовою часткою NaOH 50% (ρ = 1,53 г/см3), €кий потр≥бний дл€ приготуванн€ розчину NaOH обТЇмом 2,5 л з мол€рною концентрац≥Їю 0,2 моль/л.
«авданн€ 8.
1. ƒо 500 мл розчину з масовою часткою HCl 40% (ρ = 1,198 г/см3) додали 2,5 л води. «найд≥ть масову частку HCl у одержаному розчин≥.
2. —к≥льки грам≥в MgCl2 Ј 6Ќ2ќ треба вз€ти дл€ приготуванн€ 40 мл розчину з екв≥валентною мол€рною концентрац≥Їю 0,3 моль/л?
«авданн€ 9.
1. —к≥льки грам≥в H3–O4 необх≥дно дл€ приготуванн€ 10 мл розчину з масовою часткою ортофосфорноњ кислоти 90% (ρ = 1,74 г/см3)?
|
|
|
2. «м≥шали 20 мл розчину з масовою часткою Nа2—O3 17% (ρ = 1,18 г/см3) та 80 мл розчину з масовою часткою Nа2—O3 15% (ρ = 1,16 г/см3) та додали води до 1 л. ¬изначте мол€рну концентрац≥ю одержаного розчину.
«авданн€ 10.
1. ” 2 кг води розчинено 28,75 г ZnSO4 Ј 7H2O. ќбчисл≥ть масову частку кристалог≥драту та безводного сульфату цинку (≤≤) у розчин≥.
2. «м≥шали 3 л розчину з екв≥валентною мол€рною концентрац≥Їю 0,12 моль/л та 85 мл розчину з екв≥валентною мол€рною концентрац≥Їю 0,5 моль/л. Ќехтуючи зм≥ною обТЇму, обчисл≥ть екв≥валентну мол€рну концентрац≥ю одержаноњ сум≥ш≥.
«авданн€ 11.
1. ” 2 кг води розчинено 28,75 г ZnSO4 Ј 7H2O. ќбчисл≥ть масову частку кристалог≥драту та безводного сульфату цинку (≤≤) у розчин≥.
2. —к≥льки води та розчину ќЌ з ω = 48% (ρ = 1,49 г/см3) треба додати до 100 мл розчину ќЌ з ω = 49% (ρ = 1,5 г/см3), щоб одержати 7 л розчину з екв≥валентною мол€рною концентрац≥Їю 1,2 моль/л?
«авданн€ 12.
1. —к≥льки грам≥в Pb(NO3)2 треба розчинити у 1 кг води, щоб приготувати розчин ≥ з масовою часткою н≥трату свинцю 38%?
2. ќбчисл≥ть обТЇм розчину сол€ноњ кислоти з масовою часткою HCl 4% (ρ = 1,02 г/см3), €кий треба вз€ти дл€ приготуванн€ розчину з масовою часткою HCl 2% (ρ = 1,01 г/см3).
«авданн€ 13.
1. —к≥льки грам≥в Na2CO3 Ј 10Ќ2ќ треба вз€ти, щоб приготувати 5 л розчину з масовою часткою Na2CO3 15% (ρ = 1,16 г/см3)?
2. —к≥льки сульф≥ду амон≥ю треба вз€ти, щоб приготувати 250 мл розчину з екв≥валентною мол€рною концентрац≥Їю (NЌ4)2S 0,4 моль/л? якою Ї мол€рна концентрац≥€ цього розчину?
«авданн€ 14.
1. ¬изначте масову частку речовини у розчин≥ одержаному при зм≥шуванн≥ розчин≥в масою 24 г з масовою часткою речовини 60% та масою 140 г з масовою часткою речовини 90%.
2. ќбчислити об' Їм розчину с≥рчаноњ кислоти густиною ρ = 1,840 г/см3 та масовою часткою 96 %, необх≥дний дл€ приготуванн€ 2 л розчину масовоњ концентрац≥њ 1,92 г/л.
«авданн€ 15.
1. —к≥льки к≥лограм≥в води треба додати до 1 кг розчину з масовою часткою речовини 90%, щоб одержати розчин з масовою часткою речовини 70%?
2. ќбчислити масу хлориду натр≥ю та обТЇм води, необх≥дн≥ дл€ при-готувавнн€ 300 г розчину з масовою часткою сол≥ 5 % (густина розчину ρ = 1,001 г/см3).
«авданн€ 16.
1. ƒо €кого обТЇму необх≥дно розбавити 100 мл розчину хлороводню мол€рноњ концентрац≥њ с(Ќ—1) = 1,5 моль/л, щоб отримати розчин мол€рноњ концентрац≥њ с(Ќ—1) = 0,4 моль/л.
2. ќбчислити масову частку зал≥за в розчин≥, приготованому з 50 г сол≥ FeSO4. Ј7H2O та 450 г води.
«авданн€ 17.
1. —к≥льки к≥лограм≥в розчину з масовою часткою CuSO4 6% треба додати до 1 кг розчину з масовою часткою 15%, щоб одержати розчин ≥з масовою часткою 10%?
2. який обיЇм сол€ноњ кислоти з масовою часткою хлороводню 36,6 % та густиною ρ = 1,300 г/см3 необх≥дний дл€ приготуванн€ 1,5 л розчину екв≥валентноњ мол€рноњ концентрац≥њ 0,1 моль/л?






