1. ќтщепление галогена атомарным водородом по методу —тепанова
—Ќ3-—l + 2H  CH4 + HCl
CH4 + HCl
2C2H5OH+2Na
ћетод используетс€ дл€ количественного определени€ галогенов в органических соединени€х.
2.ќтщепление галогенов под действием металлов:
а) реакци€ ¬юрца
—H3Br + 2Na + Br-CH3  2NaBr + CH3-CH3
2NaBr + CH3-CH3
б) получение этиленовых углеводородов
—H2-CH2 + Zn  CH2=CH2 + ZnCl2
CH2=CH2 + ZnCl2
Cl Cl
в) получение циклических углеводородов
—H2-CH2-CH2 + Zn  CH2-CH2
CH2-CH2
Br Br CH2
г) под действием магни€
—2H5Br + Mg  C2H5MgBr
C2H5MgBr  C2H6
C2H6
-MgBr(OH)
III. –еакции отщеплени€ галогеноводорода
1. ѕри нагревании
—H3-CH2Cl  HCl + CH2=CH2
HCl + CH2=CH2
2. ѕод действием спиртового раствора щелочи
—H3-CH3-Cl + KOHспиртовый 
A B
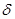 +///
+/// 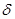 +//
+// 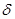 +/
+/ 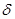 -
-
H H
H  C
C  C
C  Cl
Cl  CH2=CH2 + H2o + KCl
CH2=CH2 + H2o + KCl
HO- H H K+
R=k[A][B]
–еакци€ нуклеофильного отщеплени€ протекает по механизму ≈N2.
” третичных галоидных алкилов идет, в основном, по механизму ≈N1.
H CH3 H CH3 CH3
H-C-C-Cl  H-C-C+
H-C-C+  H+ + H2C=C
H+ + H2C=C
H CH3 H CH3 CH3
ј изобутилен
r=k[A]
“акие реакции обычно сопутствуют реакци€м нуклеофильного замещени€.
Ћ≈ ÷»я 7
√јЋќ√≈Ќќѕ–ќ»«¬ќƒЌџ≈ Ќ≈ѕ–≈ƒ≈Ћ№Ќџ’ ”√Ћ≈¬ќƒќ–ќƒќ¬
ѕодраздел€ютс€ на три типа.
I. √алогенопроизводные винилового типа: атом галогена находитс€ у непредельного атома углерода.
—Ќ2=—ЌCl хлористый винил
—Ќ2=——l2 хлористый винилиден
—Ќ2=—-—Ќ=—Ќ2 хлорпрен
Cl
II. √алогенпроизводные аллильного типа (галоген в  -положении к двойной св€зи).
-положении к двойной св€зи).
—Ќ2=—Ќ-—Ќ2-—l хлористый аллил
—Ќ3-—Ќ=—Ќ-—Ќ2Cl хлористый кротил
III. √алогенпроизводные с изолированным расположением двойной св€зи и Hal.
—Ќ2=—Ќ-—Ќ2-—Ќ2-Cl 4-хлор-1-бутен
¬ этом случае галоген не подвергаетс€ вли€нию  -св€зи и ведет себ€ так же, как в предельных галогеноводородах.
-св€зи и ведет себ€ так же, как в предельных галогеноводородах.
I. √алогенопроизводные винильного типа.
ѕолучение
1) ѕрисоединение одной молекулы ЌЌal к ацетиленовым углеводородам.
—Ќ  —Ќ + HCl
—Ќ + HCl  —Ќ2=—ЌCl
—Ќ2=—ЌCl
хлористый винил
—Ќ2=—Ќ-—  —Ќ + HCl
—Ќ + HCl  —Ќ2=—Ќ-—=—Ќ2
—Ќ2=—Ќ-—=—Ќ2
Cl
1,3-бутадиенин хлорпрен
2) ѕиролиз полигалогенопроизводных
—Ќ2-—Ќ2 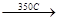 HCl + —Ќ2=—Ќ-Cl
HCl + —Ќ2=—Ќ-Cl
Cl Cl
хлористый хлористый
этилен винил
3) ƒействие недостатка спиртового раствора ќЌ на полигалогенопроизводные
Cl Cl
—Ќ-—Ќ  —ЌCl=CCl2
—ЌCl=CCl2
Cl Cl -HCl
тетрахлорэтан трихлорэтилен
ќсобенности свойств
1. —оединени€ винильного типа про€вл€ют инертность в реакци€х нуклеофильного замещени€. ѕричина заключаетс€ в особенности электронного строени€. √рафически это можно показать следующим образом:
..
—Ќ2=—Ќ-Cl:
..
или
Ќ —l
C - C p-  -сопр€жени€
-сопр€жени€
H H
’лор расположен через одну простую св€зь от двойной и имеет 3 неподеленных пары электронов. Ќаблюдаетс€ эффект p-  -сопр€жени€, т.е. перекрытие р-электронной орбитали хлора с
-сопр€жени€, т.е. перекрытие р-электронной орбитали хлора с  -орбитал€ми атома углерода. ¬ результате часть электронного облака хлора отт€гиваетс€ в сторону двойной св€зи и дипольный момент св€зи C-—l уменьшаетс€.
-орбитал€ми атома углерода. ¬ результате часть электронного облака хлора отт€гиваетс€ в сторону двойной св€зи и дипольный момент св€зи C-—l уменьшаетс€.
2. –еакции присоединени€ по двойной св€зи идут в соответствии с правилом ћарковникова, т.к.
|
|
|
 -
- 
 +..
+..
—Ќ2=—Ќ  —l + HCl CЌ3-—ЌCl2
—l + HCl CЌ3-—ЌCl2
этилиденхлорид
/+ћ/</-I/, по p- 
+ +
—Ќ3  —Ќ-Cl (1) —Ќ2-—Ќ2
—Ќ-Cl (1) —Ќ2-—Ќ2  Cl (2)
Cl (2)
Ѕолее устойчив катион (1), т.к. электродонорна€ метильна€ группа частично компенсирует положительный зар€д.
3. ќчень легко полимеризуютс€.
n —Ќ2=—ЌCl  (-CЌ2-—ЌCl-)n
(-CЌ2-—ЌCl-)n
винилхлорид ѕ¬’-самые массовые пластмассы
ѕолимеры на основе винилхлорида наход€т широчайшее применение дл€ изготовлени€ пленок, пластмассовых изделий. ќни устойчивы к действию влаги, кислот, щелочей, нефтепродуктов, обладают хорошими электроизол€ционными свойствами.






