1. “емпература кипени€ олефинов немного ниже (на 5-15о—) температуры кипени€ соответственных предельных углеводородов. ”величиваетс€ с возрастанием молекул€рного веса. ќлефины с углеродной цепью —2 Ц —4 Ц газ; —5 Ц —16 Ц жидкости; —17 и более Ц твердые вещества.
а. “кип нормальных изомеров выше, чем разветвленных;
б. “кип цис-изомеров выше, чем транс-изомеров;
в. “кип увеличиваетс€ при перемещении двойной св€зи ближе к центру.
2. “емпература плавлени€ также зависит от строени€. ќна выше у транс-изомеров и увеличиваетс€ при перемещении двой ной св€зи ближе к центру.
3. ѕлотность олефинов несколько выше, чем у парафинов (т.к. больше относительное содержание более т€желого углерода).
d204C5H12 = 0,626 d204C5H10 = 0,641
4. ќлефины раствор€ютс€ в воде незначительно, но лучше, чем парафины. –астворимость увеличиваетс€ с увеличением пол€рности углеводорода. ќлефины хорошо раствор€ютс€ в растворах солей т€желых металлов, например, в растворе CuCl (вследствие комплексообразовани€).
5. ќптические свойства. ќлефины имеют более высокий показатель преломлени€, что говорит об их более высокой пол€ризуемости.
6. ќлефины дают избирательное поглощение в инфракрасной области (1670 Ц 1560 см-1 и 100 Ц 830 см-1). ¬ ”‘-области олефины дают поглощение при 180-200 нм.
’имические свойства олефинов
’имические свойства олефинов обусловлены природой двойных св€зей.
1) ƒвойна€ св€зь состоит из неравноценных св€зей: d-св€зи и p-св€зи. p-св€зь отличаетс€ от d-св€зи меньшей прочностью и большей пол€ризуемостью. Ѕлагодар€ малой прочности p-св€зь легко разрываетс€. Ётим обуславливаетс€ способность олефинов вступать в реакции присоединени€.
2) ¬ зависимости от характера реагента и условий проведени€ реакции могут идти или по гомолитическому, или по гетеролитическому механизму.
3) Ѕлагодар€ легкой пол€ризуемости p-электронов и их способности захватывать положительные частицы. большинство реакций олефинов протекают по гетеролитическому электрофильному механизму.
–еакции присоединени€
 1. ѕрисоединение водорода происходит при действии молекул€рного водорода —Ќ2=—Ќ2 + Ќ2 Ni, Pt, Pd —Ќ3-—Ќ3. Ёта реакци€ гетерогенного катализа протекает на поверхности твердого катализатора. включает в себ€ три этапа:
1. ѕрисоединение водорода происходит при действии молекул€рного водорода —Ќ2=—Ќ2 + Ќ2 Ni, Pt, Pd —Ќ3-—Ќ3. Ёта реакци€ гетерогенного катализа протекает на поверхности твердого катализатора. включает в себ€ три этапа:
1) адсорбци€ молекул из газовой фазы на поверхности катализатора;
2) взаимодействие молекул в адсорбированном состо€нии;
3) десорбци€ продуктов реакции с поверхности катализатора.
атализаторы Ц металлы: Fe, Co, Ni, Pt, Pd, имеющие d-уровни, не полностью заполненные электронами. за счет чего могут обраховыватьс€ донорно-акцепторные св€зи с олефинами; происходит хемосорбци€ олефинов на поверхности катализатора. ћолекулы водорода тоже хемосорбируютс€ с металлами в виде свободных атомов.




 Ќ —Ќ2 —Ќ2 Ќ
Ќ —Ќ2 —Ќ2 Ќ
| Ni |
—корость реакций зависит от поверхности катализатора, величины его частиц, а также от количества активных центров на поверхности катализатора (которыми €вл€ютс€ дефекты кристаллической решетки), на них начинает€ реакци€. —корость гидрировани€ олефинов зависит от разветвленности цепи. „ем больше заместителей. тем труднее протекает реакци€ (т.к. слабее адсорбци€).
|
|
|
2. ѕрисоединение галогенов.
F2 Ц присоедин€етс€ со взрывом;
Cl Ц на рассе€нном свету или при низкой температуре в присутствии катализатора FeCl3;
Br2 Ц при комнатной температуре в пол€рных растворител€х;
I Ц медленно в пол€рных растворител€х.
ћеханизм реакций может быть и электрофильным, и свободнорадикальым.
Ёлектрофильное присоединение происходит под действием пол€рных растворителей. ћолекула галогена пол€ризуетс€ Brd+ 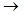 Brd- под действием Ќ2ќ. ѕри взаимодействии ее с олефинами наблюдаетс€ последовательноеобразование p-комплекса и d-комплекса.
Brd- под действием Ќ2ќ. ѕри взаимодействии ее с олефинами наблюдаетс€ последовательноеобразование p-комплекса и d-комплекса.
CH2=CH2 + Brd+ 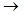 Brd-
Brd- 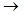 CH2=CH2
CH2=CH2 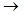 CH2-CH2 + Br
CH2-CH2 + Br  CH2-CH2
CH2-CH2
Brd+  Brd- Br Br Br
Brd- Br Br Br
p-комплекс d-комплекс 1, 2-дибромэтан
p-комплекс образуетс€ за счет захвата p-электронами положительного конца дипол€ молекулы галогена, затем образуетс€ d-комплекс за счет обобщени€ атомом брома p-электронной пары с образованием d-св€зи. ќдновременно происходит отщепление аниона брома. «атем карбокатион присоедин€ет анион брома. “аким образом, наблюдаетс€ ступенчатое присоединение двух атомов галогена.
—вободнорадикальное присоединение наблюдаетс€ при действии газообразного галогена в отсутствии растворител€ на свету или при нагревании.
»: инициирование Cl2  2Cl
2Cl
÷: цепь CH2=CH2 + Cl.  CH2-CH2.
CH2-CH2.
Cl
CH2-CH2. + Cl2  CH2-CH2 + Cl.
CH2-CH2 + Cl.
Cl Cl Cl
ќ: обрыв цепи CH2-CH2. + Cl.  CH2-CH2
CH2-CH2
Cl Cl Cl
3. ѕрисоединение галогеноводородов
Ћегкость присоединени€ возрастает от Ќ—l HI, т.к. увеличиваетс€ пол€ризуемость молекул реагента. ЌF вызывает реакции полимеризации, его присоединение наблюдаетс€ только при назких темпратурах. ћеханизм реакций Ц электрофильный. HCl  H++ Cl-.
H++ Cl-.
ћолекула олефина легко захватывает электрофильный реагент Ќ+ p-электронами (образуетс€ p-комплекс), затем происходит образование
d-св€зи с Ќ+ за счет p-электронов (образуетс€ d-комплекс),
CH2=CH2 + H+  CH2=CH2
CH2=CH2  CH3-CH2+
CH3-CH2+  CH3CH2Cl
CH3CH2Cl
H+
 -комплекс
-комплекс  -комплекс хлористый этил
-комплекс хлористый этил
(карбкатион)
который стабилизируетс€ за счет присоединени€ аниона хлора.
≈сли молекула содержит при ненасыщенном углероде какой-либо заместитель —Ќ2=—Ќ-’, то место присоединени€ Ќ+ определ€етс€ природой заместител€. ¬се заместители дел€тс€ на две группы: электронодонорные (несущие избыток электронов и подающие их в цепь) и электроноакцепторные (имеющие недостаток электронов и прит€гивающие к себе p-электроны двойной св€зи).
¬се углеводородные радикалы: —Ќ3, —2Ќ5 и т.д. €вл€ютс€ группами электронодонорными,
H
 —
—  Ќ
Ќ
H
и отталкивают подвижные p-электроны двойной св€зи.
Ќапример:
CH2 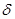 -=—Ќ
-=—Ќ 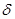 +
+  —Ќ3
—Ќ3
пропилен
¬ результате наиболее высока€ электронна€ плотность создаетс€ у группы —Ќ2; к ней и будет присоедин€тьс€ протон.
CH2 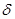 -=—Ќ
-=—Ќ  —H3 + H+
—H3 + H+  CH3-CH+-CH3
CH3-CH+-CH3  CH3-CH-CH3
CH3-CH-CH3
Cl
карбкатион хлористый изопропил
“аким образом, присоединение галогеноводородов к олефинам идет по правилу ћарковникова: водород присоедин€етс€ к наиболее гидрированному атому углерода.
Ёффект араша
ѕрисоединение галогеноводородов в присутствии примесей перекисей происходит не по правилу ћарковникова. ѕричина Ц свободнорадикальный механизм реакции.
HO: OH  2HO
2HO
HO. + HBr  H2O + Br
H2O + Br
Br. + CH2=CH-CH3  CH2Br-CH.-CH3 (1)
CH2Br-CH.-CH3 (1)
CH2.-CHBr-CH3 (2)
Ѕолее устойчив (1) и образуетс€ преимущественно, т.к. свободный электрон сопр€жен с 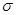 -электронами п€ти —-Ќ св€зей.
-электронами п€ти —-Ќ св€зей.
|
|
|
—H2Br-CH.-CH3 + HBr  CH2Br-CH2-CH3 + Br
CH2Br-CH2-CH3 + Br
Ёлектрофильные реакции протекают через промежуточное образование карбкатионов, поэтому рассмотрим их свойства.
—войства карбкатионов
1. арбкатионы образуютс€ в пол€рных средах под действием пол€ризованных реагентов по схемам:
R3C:H + Br+  HBr + R3C+
HBr + R3C+
CH2=CH2 + Br+  CH2+-CH2Br
CH2+-CH2Br
2. ”стойчивость карбкатионов увеличиваетс€ от первичного к третичному
CH3  C+
C+  CH3 > CH3
CH3 > CH3  CH+
CH+  CH3 > CH3
CH3 > CH3  CH2+
CH2+
CH3
ѕричина Ц увеличение количества элетродонорных групп, окружающих зар€женный атом углерода.
3. ’имические превращени€ карбкатионов.
а) соединение с анионом: R+ + A-  RA
RA
б) отщепление протона (от наименее гидрированного из 2-х соседних звеньев:
CH3-C+-CH2-CH3 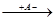 CH3-C=CH-CH3 + HA
CH3-C=CH-CH3 + HA
CH3 CH3
2-метил-2-бутен
в) присоединение к непредельному углеводороду:
R+ + CH2=CH2  R-CH2-CH2+
R-CH2-CH2+
–ассмотрим остальные химические свойства олефинов.
4. ѕрисоединение Ќ2SO4 к олефинам происходит в соответствии с правило ћарковникова, т.к. реагент очень пол€рный, и реакци€ всегда идет по ионному электрофильному механизму.
HOSO2OH 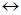 H+ + O-SO2OH
H+ + O-SO2OH
CH2 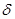 -=CH
-=CH  CH3 + H+
CH3 + H+  CH3
CH3  CH+
CH+  CH3
CH3 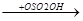 CH3-CH-CH3
CH3-CH-CH3
OSO2OH
более устойчив, чем первичный изопропиловый эфир
серной кислоты
–еакци€ присоединени€ Ќ2SO4 идет тем легче, чем больше радикалов окружает остаток этилена, т.е. чем больше электронна€ плотность у ненасыщенных атомов углерода.
 “ак, —Ќ2=—Ќ2 (этилен) Ц присоедин€ет только концентрированную Ќ2SO4; —Ќ2=—Ќ-—Ќ3 (пропилен) Ц присоедин€ет 78% кислоту; —Ќ2=—-—Ќ3 (изобутилен) Ц присоедин€ет 65% кислоту. —Ќ3
“ак, —Ќ2=—Ќ2 (этилен) Ц присоедин€ет только концентрированную Ќ2SO4; —Ќ2=—Ќ-—Ќ3 (пропилен) Ц присоедин€ет 78% кислоту; —Ќ2=—-—Ќ3 (изобутилен) Ц присоедин€ет 65% кислоту. —Ќ3
5. ѕрисоединение воды требует катализатора и также происходит только в соответствии с правилом ћарковникова.
—H2 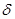 -=CH
-=CH 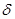 +
+  CH3 + H+OH
CH3 + H+OH  CH3-CHOH-CH3
CH3-CHOH-CH3
ѕропилен »ѕ—
6. √ипогалоидирование Ц присоединение гипогалогентных кислот Ќќ√ал. (√ал2 в присутствии щелочи).
Cl: Cl + Na+OH-  NaCl + HO-Cl+ хлорноватиста€ кислота
NaCl + HO-Cl+ хлорноватиста€ кислота
 HO-Cl+ + CH3
HO-Cl+ + CH3  CH
CH 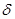 +=CH2
+=CH2 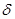 - CH3-CH-CH2
- CH3-CH-CH2
OH Cl
ѕропиленхлоргидрин
–еакции окислени€
Ѕлагодар€ легкости разрыва p-св€зи олефины хорошо окисл€ютс€. –азличаютс€ следующие виды реакций окислени€:
1. — разрывом только p-св€зи.
а) окисление по ¬агнеру Ц действие разбавленного водного раствора KMnO4 при комнатной температуре Ц приводит к образованию гликолей
—H2=CH2 + O  CH2-CH2 + HOH
CH2-CH2 + HOH  CH2-CH2
CH2-CH2
(KMnO4) O OH OH
+H2O
окись этилена этиленгликоль
б) окисление надкислотами (реакци€ ѕрилежаева) приводит к образованию окисей.
O
—H2=CH2 + C6H5-C-O-OH  CH2-CH2 + C6H5C
CH2-CH2 + C6H5C
O O OH
надбензойна€ кислота бензойна€ кислота
2. ќкисление с разрывом углеродной цепи происходит при действии сильных окислителей и при нагревании, например:
KMnO4 + H2SO4; продуктами реакций могут быть кетоны и карбоновые кислоты
O O
CH3-C=CH-CH3 + 3O  CH3-C + CH3-C
CH3-C + CH3-C
CH3 CH3 OH
2-метил-2-бутен
3. ќзонирование Ц окисление озоном ќ3.
ѕри действии озона сначала происходит его присоединение по двойной св€зи с образованием озонидов.
O-O-O O Ц O CH3
—H2=C-CH3 + O3  CH2-C-CH3
CH2-C-CH3  CH2 C
CH2 C
CH3 CH3 O CH3
ќзониды разлагаютс€ водой, дава€ альдегиды и кетоны, в зависимости от наличи€ заместителей
O-O-O O O
CH2-C-CH3 + H2O  H2O2 + CH2 + C-CH3
H2O2 + CH2 + C-CH3
CH3 CH3
ѕо строению образующихс€ соединений можно легко установить местоположение двойной св€зи в олефине.
–еакции полимеризации
Ёто соединение нескольких или многих молекул в одну без изменени€ состава, происход€щее за счет разрыва двойных св€зей. —уществует два вида полимеризации олефинов:
1. —тупенчата€ полимеризаци€ Ц происходит в присутствии катализаторов (H2SO4, AlCl3), приводит к образованию димеров, тримеров, тетрамеров и т.д., причем на каждой стадии полученный продукт выдел€етс€ как устойчивое соединение. –еакци€ идет по механизму электрофильного присоединени€
CH3  C=CH2
C=CH2 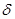 - + H+O-SO2OH
- + H+O-SO2OH  CH3-C+-CH3 + CH2
CH3-C+-CH3 + CH2 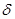 -=C
-=C  CH3
CH3 
CH3 конц. CH3 —H3
|
|
|
»зобутилен
—H3 CH3 CH3
 CH3-C-CH2-C+-CH3
CH3-C-CH2-C+-CH3  CH3-C-CH=C-CH3
CH3-C-CH=C-CH3  —H3-C-CH2-CH-CH3
—H3-C-CH2-CH-CH3
CH3 CH3 -H+ CH3 CH3 H2 CH3 CH3
ƒимер изобутилена изооктан
(октановое число прин€то за 100)
ќбразовавшийс€ димер может снова реагировать с катализатором и затем с олефином, в результате чего образуетс€ тример и т.д. »спользуетс€ дл€ получени€ жидких высокооктановых углеводородов.
2. ÷епна€ полимеризаци€ протекает под действием инициаторов или катализаторов по цепному механизму. Ќизкомолекул€рные продукты полимеризации не могут быть выделены, т.к. не фвл€ютс€ устойчивыми веществами. –еакци€ приводит к образованию высокомолекул€рных соединений.
nR1-CH=CH-R2  (-CH-CH-)n
(-CH-CH-)n
R1 R2
где n Ц степень полимеризации, котора€ составл€ет дес€тки и сотни тыс€ч. »сходное соединение носит название мономер. ¬ысокомолекул€рный продукт полимеризации Ц полимер. ѕолимеры низкого молекул€рного веса (где n не более нескольких тыс€ч) называютс€ олигомеры. –еакции цепной полимеризации могут протекать как по свободно-радикальному, так и по ионному механизму.
ѕримером свободно-радикальной полимеризации €вл€етс€ получение оксиэтилена высокого давлени€ (способ открыт в 1933 г.).
NCH2=CH2 + 0,1% O2 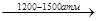 (-CH2-CH2-)n
(-CH2-CH2-)n
190-2100C
ислород способствует инициированию реакции за счет образовани€ перекисных радикалов. ≈сли обозначить эти радикалы, инициирующие реакцию R, то механизм реакции будет выгл€деть так:
R. + CH2=CH2  R-CH2-CH2.
R-CH2-CH2. 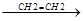 R-CH2-CH2-CH2-CH2.
R-CH2-CH2-CH2-CH2. 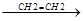
и т.д. до обрыва цепи
»онна€ цепна€ полимеризаци€ наблюдаетс€ в присутствии металлов. металлических катализаторов. катализаторов системы ÷иглера*. ѕример Ц получение полиэтилена низкого давлени€.
NCH2=CH2  (-CH2-CH2-)n
(-CH2-CH2-)n
R=1-10 атм
T0=-70+150C
Ј ћеханизм действи€ катализатора ÷иглера. ѕол€рна€ св€зь —-Al катализатора легко разрушаетс€ гетеролитически.
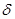 -
- 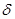 +
+
+CH2=CH
CH3
(—2Ќ5)Al+  C2-H5 + C
C2-H5 + C 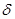 -H2=C
-H2=C 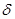 +H
+H  (C2H5)2Al+-CH2--CH-C2H5
(C2H5)2Al+-CH2--CH-C2H5 
CH3 CH3
 (C2H5)2Al-CH2-CH-CH2-CH-C2H5
(C2H5)2Al-CH2-CH-CH2-CH-C2H5
CH3 CH3
ѕолимеры, образованные в результате ионной полимеризации, отличаютс€ правильным (регул€рным) химическим и пространственным строением цепи и обладают большей кристалличностью, жесткостью и прочностью.
¬се эти полимеры €вл€ютс€ по существу предельными углеводородами чрезвычайного высокого молекул€рного веса. ак и все предельные углеводороды, они устойчивы к окислител€м. к действию концентрированных кислот и других агрессивных реагентов.
“еломеризаци€ Ц разновидность цепной полимеризации, отличаетс€ тем, что процесс проводитс€ с искусственным ранним обрывом цепи на стадии образовани€ низкомолекул€рных полимеров, содержащих 3-4 молекулы мономера. —остав продуктов теломеризации отличаетс€ от состава мономера, т.к. часть молекул растворител€, вызывающих обрыв цепи, входит в молекулу теломера. Ќапример, теломеризаци€ этилена в присутствии ——l4.
R. + CCl4  RCl + .CCl3 . CCl3 + CH2=CH2.
RCl + .CCl3 . CCl3 + CH2=CH2.  CCl3-CH2-CH2.
CCl3-CH2-CH2.
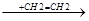 CCl3-CH2-CH2-CH2-CH2.
CCl3-CH2-CH2-CH2-CH2. 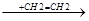
CCl3-CH2-CH2-CH2-CH2-CH2-CH2. 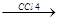 .CCl3 + CCl-(CH2-CH2)3-Cl
.CCl3 + CCl-(CH2-CH2)3-Cl






