Высший химический колледж РАН
Федеральное государственное бюджетное учреждение науки
Институт органической химии
Им. Н.Д. Зелинского Российской академии наук
(ИОХ РАН)
Лаборатория химии диазосоединений №6
Александр Юрьевич Белый
Курсовая работа
Синтез п-нитробензоилазида и изучение его реакции с гепта(метоксикарбонил)циклогептатриенил анионом
Научный руководитель:
асп., инж.-иссл. Саликов Ринат Фаритович
Москва, 2013
Оглавление
Оглавление.................................................................................................................................... 2
ВВЕДЕНИЕ................................................................................................................................... 3
Литературный обзор..................................................................................................................... 4
Обсуждение результатов.............................................................................................................. 7
Экспериментальная часть........................................................................................................... 11
ВЫВОДЫ..................................................................................................................................... 14
Список литературы..................................................................................................................... 15
ВВЕДЕНИЕ
Ранее в нашей лаборатории была обнаружена уникальная каскадная реакция диметилброммалеата (диметил-2,3-дибромсукцината) с метилдиазоацетатом в пиридине, в результате которой в одну стадию получается гептаметиловый эфир циклогепта-1,3,5-триен-1,2,3,4,5,6,7-гептакарбоновой кислоты (ГМЦГ). Последний легко депротонируется под действием оснований с образованием стабильного циклогептатриенильного аниона. Было показано, что ГМЦГ и его анион способны вступать в реакции с широким спектром соединений, часто сопровождающиеся значительными перестройками углеродного скелета. На основе изученных трансформаций был предложен ряд оригинальных синтезов [1].
Известно, что алкены способны вступать в реакцию 1,3-диполярного присоединения с азидами, причём электронодефицитные алкены реагируют особенно легко. Данный тип трансформаций относят к так называемым “click” реакциям, суть которых заключается в эффективном построении молекул из отдельных блоков. Данные реакции находят широкое применение в медицинской химии [2].
В связи с тем, что с одной стороны ГМЦГ и его анион являются высоко реакционноспособными соединениями, о чём упоминалось выше, а с другой – азиды склонны реагировать с электронодефицитными кратными связями, было решено исследовать взаимодействие ГМЦГ-аниона с азидами.
К настоящему моменту, в рамках данного проекта, уже были изучены реакции аниона с некоторыми азидами, и было показано, что наиболее легко реакция протекает с участием азидов, содержащих электроно-акцепторные группы. Таким образом, целью настоящей работы является синтез 4-нитрокарбонилазида и изучение его реакции с гепта(метоксикарбонил)циклогептатриенилкалием.
Литературный обзор
Впервые реакция 1,3-диполярного присоединения органических азидов к ацетиленам была описана более ста лет назад. Несколько позднее было показано, что алкены также способны вступать в аналогичную реакцию [3]. Поскольку реакция протекает при высокой температуре, то в её результате образуется смесь 1,4- и 1,5-региоизомеров (схема 1).
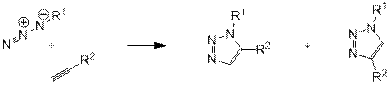
Схема 1.
Проблема низкой региоселективности была решена в группе Шарплесса путём использования комплексов Cu(I), генерируемых in situ из более доступных солей Cu(II) восстановлением под действием аскорбиновой кислоты (схема 2), при этом наблюдается образование только 1,4-региоизомера. Так, при взаимодействии бензилазида с пропаргилфениловым эфиром образуется 1,4-дизамещённый триазол [4].

Схема 2.
Не так давно Родионов и др. показали, что данная реакция ускоряется в присутствии третичных аминных лигандов [5]. Например, реакция фенилацетилена с бензилазидом ускоряется на несколько порядков в присутствии лиганда (схема 3).
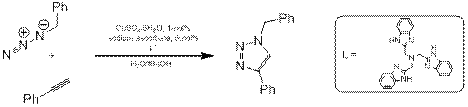
Схема 3.
Было показано, что в зависимости от используемого катализатора реакцию удаётся направить как в сторону образования 1,4-региоизомера, так и в сторону 1,5-региоизомера (схема 4). Так, при взаимодействии азидного производного рибозы с терминальными алкинами в случае использования медного катализатора образуется 1,4-дизамещённое производное, в случае же рутениевого катализатора (Cp*Ru(PPh3)2Cl) образуется 1,5-производное [6].
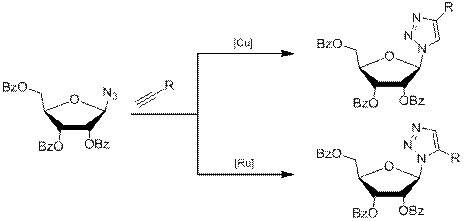
Схема 3.
В данную реакцию способны вступать не только алкил, но также ацилазиды и сульфонилазиды [6]. Примечательно, что в данных реакциях образуются преимущественно 1,5-региоизомеры, что видимо связанно с электронным эффектом заместителя в азиде (схема 5).
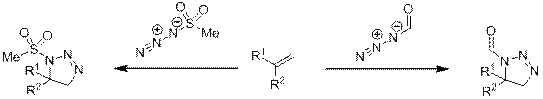
Схема 5.
Так же было показано, что тозилазид при наличии двух конкурирующих двойных связей в молекуле легче присоединяется к электронодефицитной связи [7] (схема 6). В данном случае, кроме того, происходит отщепление фурана по механизму ретро Дильса-Альдера.
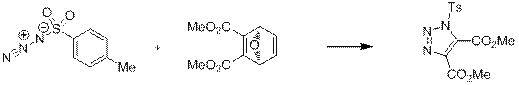
Схема 6.
Синтез триазолов помимо циклоприсоединения к алкинам может быть так же осуществлён реакцией азидов с алкенами, имеющими уходящую группу [8] (схема 7).

Схема 7.
Сами триазолины способны отщеплять азот, образуя азиридины [9] (схема 8). Причём соотношение продуктов в данном случае зависит от температуры и времени реакции, что подтверждает образование азиридина именно через триазолины.
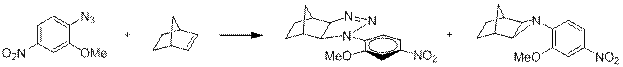 Схема 8.
Схема 8.
Как было показано в другой работе [10], образующиеся азиридины способны вступать в дальнейшие реакции. Так, в ниже приведённом примере происходит расширение цикла (схема 9).
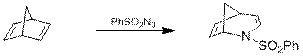
Схема 9.
Обсуждение результатов
Недавно в нашей лаборатории было обнаружено что ГМЦГ-анион (1) вступает в реакцию с мезилазидом (схема 1), образуя производное циклопентадиенильного аниона 2 с количественным выходом, проявившее в ходе исследований ряд интересных свойств, которые изучаются в данное время.
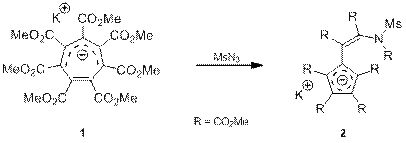
Схема 1.
С целью изучения данной реакции, а так же установления влияния структуры продуктов на их свойства, было решено провести её с рядом других азидов. Было показано, что тозилазид реагирует с анионом также с высоким выходом с образованием аналогичного продукта. Однако оказалось, что при взаимодействии циклопропанкарбонилазида с анионом выход конечного продукта составляет всего 29%. В данной работе представлено изучение реакции ГМЦГ-аниона с азидом п -нитробензойной кислоты.
Данный азид был получен в три стадии из коммерчески доступного и имеющегося в наличии п-нитробензальдегида (3). На первой стадии альдегид 3 был окислен в кислоту 4 перманганатом калия [12], при этом выход составил 95% (схема 2). Завершение реакции определяли по исчезновению характерной окраски перманганат-иона, время реакции составило несколько минут.
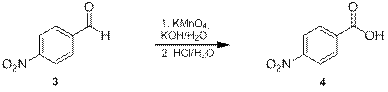
Схема 2.
Далее из кислоты 4 был получен хлорангидрид 5 путём её кипячения в избытке тионилхлорида [12] (схема 3), завершение реакции определяли по прекращению активного газовыделения и гомогенизации смеси.
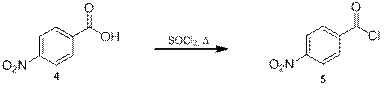
Схема 3.
На следующей стадии из хлорангидрида 5 был получен азид 6 взаимодействием с азидом натрия в безводном ацетоне [11] (схема 4) с выходом 96%. Суммарный выход за 3 стадии составил 91%.
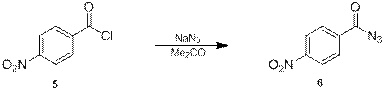
Схема 4.
Примечательно, что протонные спектры трех полученных соединений различаются, что позволяет установить чистоту соединений, однако характер спиновой системы не позволяет установить структуру соединений (рис. 1). Таким образом, структура полученных соединений была подтверждена на основании данных масс-спектрометрии.
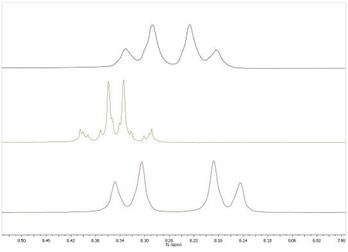
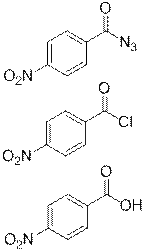
Рисунок 1.
Полученный таким образом азид вводился во взаимодействие с ГМЦГ-анионом (1) в ацетонитриле (схема 5). Ход реакции контролировали посредством спектроскопии ЯМР. Причём в процессе реакции и выделения наблюдался частичный гидролиз продукта 7 с образованием амида 8.
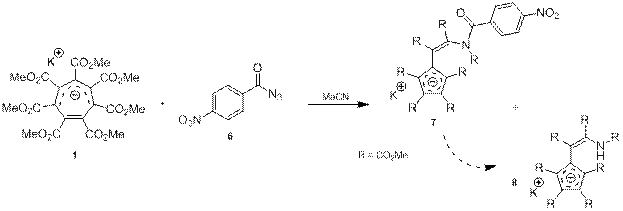 Схема 5.
Схема 5.
При этом структура соединений 7 и 8 определялась на основании спектров ЯМР 1H и ЯМР 13C, которые аналогичны спектрам ранее синтезированного аниона 2, структура которого была установлена с помощью рентгеноструктурного анализа.
Предполагаемый механизм реакции имеет вид, представленный на схеме 6. На первой стадии происходит 1,3-диполярное присоединение азида 6 к ГМЦГ-аниону 1. Образующийся триазолин 9 отщепляет азот, давая азиридин 10, который в свою очередь вступает в перегруппировку, сопровождаемую расширением цикла с образованием азациклооктатриениланиона 11. Далее соединение 11 перегруппировывается в бицикл 12, который затем раскрывается, давая анион 13. На последней стадии происходит миграция сложноэфирной группы с образованием ароматического аниона 7.
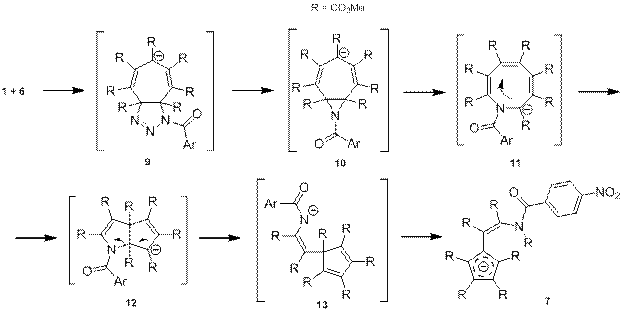
Схема 6.
Перенос сложноэфирной группы ранее был упомянут в литературе лишь однажды [13], однако недавно в нашей лаборатории был синтезирован бицикл 14, аналогичный интермедиату 12, который вступал в похожую перегруппировку, сопровождающуюся переносом сложноэфирной группы (схема 7). Именно эта реакция послужила основанием для вышеописанного механизма.
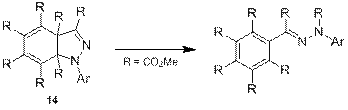
Схема 7.
Таким образом, в ходе исследования было показано, что п-нитробензоилазид реагирует с ГМЦГ-анионом хуже, чем азиды сульфокислот, кроме того, наблюдается заметный гидролиз продукта в ходе реакции и во время выделения, отсюда можно сделать вывод что данную реакцию при соответствующем подборе условий можно рассматривать как метод синтеза соединения 8, альтернативный синтез которого представляется весьма затруднительным.
Экспериментальная часть
Спектры ЯМР 1Н и 13С регистрировали на спектрометрах «Brüker AC-200» (200.13 и 50.3 МГц) и «Bruker AC-300» (300 и 75.5 МГц соответственно) для растворов в CDCl3, (CD3)2SO, CD3OD или CD3CN содержащих 0.05% Me4Si в качестве внутреннего стандарта. Масс-спектры регистрировали на приборах «Finnigan MAT INCOS-50» (ЭУ, 70 эВ, прямой ввод). Масс-спектры высокого разрешения (HRMS) регистрировали на приборе BrukermicrOTOF II. Тонкослойную хроматографию проводили на пластинках Silicagel 60 («Merck»). Для препаративных разделений использовали хроматографию на колонке (силикагель 60, 0.040–0.063 мм, «Merck») при соотношении вещество–сорбент, равном ~1: 100. В реакциях использовали стандартные продажные реактивы и химически чистые перегнанные растворители.
4-Нитробензойная кислота.
В коническую колбу объемом 50мл, снабженную магнитной мешалкой, помещают раствор перманганата калия (1.16 г, 7.3 ммоль) в 10 мл воды. Затем к раствору при перемешивании прибавляют гидроксид калия (0.2 г, 3.7 ммоль) после чего прибавляют 4-нитробензальдегид (1.75 г, 11 ммоль). Реакционную массу перемешивают 15 мин, после чего разбавляют водой и фильтруют на фильтре Шотта, двуокись марганца промывают водой. К фильтрату добавляют 5 мл конц. соляной к-ты, при этом выпадает обильный белый осадок 4-нитробензойной к-ты. Кислоту отфильтровывают на фильтре Шотта затем просушивают, выход 1.82 г (95%).
Спектр ЯМР 1H (200 МГц, DMSO-d6) d 8.17 (д, 2H, 3J = 8.8 Гц), 8.33 (д, 2H, 3J = 8.8 Гц).
Масс-спектр (ЭУ), m/z (Iотн.(%)): 167 (100) [M]+, 150 (1) [M-OH]+, 137 (16) [M-NO]+, 121 (53) [M-NO2]+, 109 (25), 104 (15) [M-OH-NO2]+.
Хлорангидрид 4-нитробензойной кислоты.
В круглодонную колбу объемом 10 мл, снабженную магнитной мешалкой и обратным холодильником помещают 4-нитробензойную к-ту (1.5 г, 9 ммоль) и тионилхлорид (1.96 мл, 3.21 г, 27 ммоль). Полученную суспензию кипятят в течение 3 часов, в результате чего смесь гомогенизируется. Избыток тионилхлорида отгоняют на роторном испарителе. Полученный хлорангидрид используется далее без дополнительной очистки.
Спектр ЯМР 1H (200 МГц, CDCl3) d 8.28‒8.42 (м).
Азид 4-нитробензойной кислоты.
В круглодонною колбу объемом 50 мл, снабженную магнитной мешалкой, счетчиком пузырьков и краником с подведенным к нему аргоном, помещают азид натрия (0.88 г, 13.5 ммоль) и 13 мл сухого ацетона, затем к полученной суспензии приливают раствор хлорангидрида 4-нитробензойной кислоты (1.67 г, 9 ммоль) в 5 мл сухого ацетона, реакционную массу перемешивают в течении полутора часов, затем фильтруют на фильтре Шотта, и упаривают на роторном испарителе. Осадок растворяют в хлористом метилене и фильтруют через вату, затем упаривают на роторном испарителе. Продукт перекристаллизовывают из петролейного эфира.
Спектр ЯМР 1H (200 МГц, CDCl3) d 8.20 (д, 2H, 3J = 8.7 Гц), 8.31 (д, 2H, 3J = 8.7 Гц).
Спектр ЯМР 13C (50 МГц, CDCl3) d 123.9 (C(3), C(5)), 130.7 (C(2), C(6)), 135.8 (C(1)), 151.7 (C(4)), 170.9 (CO).
Масс-спектр (ЭУ), m/z (Iотн.(%)): 192 (1) [M]+, 150 (100) [M-N3]+, 120 (3) [M-NO-N3]+,, 104 (45) [M-N3-NO2]+.
Реакция с ГМЦГ анионом.
Синтез проводился в атмосфере аргона. В круглодонную колбу объёмом 25 мл, снабжённую магнитной мешалкой и обратным холодильником, помещают 15 мл сухого ацетонитрила, затем туда же присыпают калиевую соль ГМЦГ (0.3 г, 0.56 ммоль) и азид 4-нитробензойной кислоты (0.16 г, 8.4 ммоль). Реакционную массу кипятят в течение 21 ч, контролируя ход реакции посредством спектроскопии ЯМР. По завершении реакции ацетонирил упаривают на роторном испарителе, а продукты выделяют при помощи колоночной хроматографии в системе хлороформ/этанол-4/1. Выход продуктов 7 и 8 составил 10% и 23% соответственно.
(Z)-1-(1,2-бис(метоксикарбонил)-2-(N-(метоксикарбонил)-4-нитробензамидо)-этен-1-ил)-2,3,4,5-тетракис(метоксикарбонил)циклопентадиенилкалий (7). Спектр ЯМР 1H (300 МГц, CD3CN) δ 8.09 (d, J = 8.8 Hz, 2H), 7.59 (d, J = 8.8 Hz, 2H), 3.79 (s, 3H), 3.66 (s, 3H), 3.64 (s, 6H), 3.54 (s, 3H), 3.49 (s, 6H).
HRMS (ESI-TOF) m/z: [M]- Вычислено для C28H25N2O17 (%): 661.1148. Найдено (%): 661.1149.
(Z)-1-(1,2-бис(метоксикарбонил)-2-(метоксикарбониламино)-этен-1-ил)-2,3,4,5-тетракис(метоксикарбонил)циклопентадиенилкалий (8). Спектр ЯМР 1H (200 МГц, CD3CN) δ 6.90 (s, 1H), 3.75 (s, 3H), 3.66 (s, 6H), 3.59 (s, 3H), 3.53 (s, 9H).
HRMS (ESI-TOF) m/z: [M]- Вычислено для C21H22NO14 (%): 512.1035. Найдено (%): 512.1051.
ВЫВОДЫ
1. Синтезирован 4-нитробензоилазид в три стадии из коммерчески доступного 4-нитробензальдегида с суммарным выходом 91%.
2. Изучена реакция 4-нитробензоилазида с анионом 1,2,3,4,5,6,-гепта(метоксикарбонил)циклогептатриена. Установлено что паранитробензилазид реагирует с ГМЦГ анионом, однако выход составляет лишь 33%, причём продукт реакции склонен к гидролизу.
Список литературы
1. Платонов Д. Н. 1,2,3,4,5,6,7-Гепта(метоксикарбонил)циклогептатриен: получение, свойства и использование в синтезе полифункциональных карбо- и гетероциклических соединений: дис. … канд. хим. наук: 02.00.03 / Платонов Дмитрий Николаевич. ‒ М., 2012.
2. Kolb H. C. Click Chemistry: Diverse Chemical Function from a Few Good Reactions / Kolb H. C., Finn M. G., Sharpless K. B. // Angew. Chem. Int. Ed. ‒ 2001. ‒ V. 40. ‒ P. 2004‒2021.
3. Huisgen R. 1,3-Dipolar cycloadditions / Huisgen R. // Proceedings of the Chemical Society. – 1961. – V. 1961. – P. 357–396.
4. Rostovtsev V. V. A stepwise huisgen cycloaddition process: copper (I)‐catalyzed regioselective “ligation” of azides and terminal alkynes / Rostovtsev V. V., Green L. G., Fokin V. V., Sharpless K. B. //Angewandte Chemie. – 2002. – V. 114. – №. 14. – P. 2708–2711.
5. Rodionov V. O. Ligand-accelerated Cu-catalyzed azide-alkyne cycloaddition: A mechanistic report / Rodionov V. O., Presolski S. I., Díaz Díaz D., Fokin V. V., Finn M. G. // Journal of the American Chemical Society. – 2007. – V. 129. – №. 42. – P. 12705–12712.
6. Schilling C., Jung N., Bräse S. Cycloaddition Reactions with Azides: An Overview // Organic Azides: Syntheses and Applications / eds Bräse S., Banert K. Chichester, UK: John Wiley & Sons, Ltd, 2010. P. 269–284.
7. Fisera L. Site-selectivity of 1,3-dipolar cycloadditions to 2,3-dimethoxycarbonyl-7-oxabicyclo[2,2,1]heptadiene / Fisera L., Povazanec F., Zalupsky P., Kovac J., Pavlovic D. // Collect. Czech. Chem. Commun. – 1983. – V. 48. – P. 3144–3153.
8. Amantini D. Synthesis of 4-Aryl-1H-1,2,3-triazoles through TBAF-Catalyzed [3 + 2] Cycloaddition of 2-Aryl-1-nitroethenes with TMSN3 under Solvent-Free Conditions / Amantini D., Fringuelli F., Piermatti O., Pizzo F., Zunino E., Vaccaro L. // J. Org. Chem. – 2005. – V. 70. – P. 6526–6529.
9. Bräse S. Organic azides: An exploding diversity of a unique class of compounds / Bräse S., Gil C., Knepper K., Zimmermann V. // Angew. Chem. Int. Ed. – 2005. – V. 44. – P. 5188–5240.
10. Emelda N. Ring expansion of substituted norbornadienes for the synthesis of mono-and disubstituted 2-azabicyclo[3.2.1]octadienes / Emelda N., Bergmeier S. C. // Tetrahedron lett. – 2008. – V. 49. – P. 5363–5365.
11. Froyen P. A particularly convenient one-pot synthesis of N-alkoxycarbonyl, N-acyl and N-aroyl substituted iminophosphoranes; improved preparation of azidoformates, aroyl and alkanoyl azides; an alternative route to complex amides / Froyen P. //Phosphorus, Sulfur, and Silicon and the Related Elements. – 1993. – V. 78. – P. 161–171.
12. Otevrel J. Investigating the spectrum of biological activity of ring-substituted salicylanilides and carbamoylphenylcarbamates / Otevrel J., Mandelova Z., Pesko M., Guo J., Kralova K., Sersen F., Jampilek J. // Molecules. – 2010. – V. 15. – P. 8122–8142.
13. Михайлов И. Е. Реакция 5-нитро-1,2,3,4,5-пентаметоксикарбонилциклопентадиена с азидом натрия / Михайлов И. Е., Душенко Г. А., Минкин В. И. // ЖОХ. – 1993. – Т. 29. – С. 1072–1073.






