ƒо реакц≥й обм≥ну в розчинах належить взаЇмод≥€ м≥ж кислотами ≥ основами, в результат≥ €коњ утворюЇтьс€ с≥ль ≥ вода. “ак≥ реакц≥њ називаютьс€ реакц≥€ми нейтрал≥зац≥њ. ¬они йдуть до к≥нц€ т≥льки тод≥, коли реагують сильн≥ основи ≥ кислоти. Ќаприклад:
HCl + KOH Ѓ KCl + H2O;
H+ + Cl- +K+ + OH- Ѓ K+ + Cl- + H2O;
H+ + OH- Ѓ H2O.
—корочене ≥онне р≥вн€нн€ показуЇ, що р≥вновага практично повн≥стю зм≥щуЇтьс€ в сторону утворенн€ води. Ќейтрал≥зац≥€ кислот ≥ основ, р≥зних за силою, до к≥нц€ не в≥дбуваЇтьс€. Ќаприклад:
CH3COOH + KOH ЂCH3COO + H2O;
CH3COOH + OH- ЂCH3COO- + H2O;
HCl + NH4OH ЂNH4Cl + H2O;
H + + NH4OH ЂNH4+ + H2O.
÷≥ реакц≥њ оборотн≥, тому що малодисоц≥йован≥ сполуки утворюютьс€ в результат≥ пр€моњ ≥ зворотноњ реакц≥й.
–еакц≥€, зворотна реакц≥€ нейтрал≥зац≥њ, тобто взаЇмод≥€ сол≥ з водою з утворенн€м кислоти ≥ основи, називаЇтьс€ г≥дрол≥зом сол≥.
ѕричиною г≥дрол≥зу Ї участь у реакц≥њ слабкоњ основи ≥ кислоти. ѕри цьому кат≥он сол≥ (в≥дпов≥дний слабк≥й основ≥) пол€ризуЇ молекулу води ≥ звТ€зуЇ г≥дроксильний ≥он. ¬изволений ≥он Ќ+ утворюЇ кисле середовище (рЌ < 7). Ќавпаки, ан≥он сол≥, в≥дпов≥дний слабк≥й кислот≥, звТ€зуЇ ≥он Ќ+ води ≥ визвол€Ї ≥он ќЌ- (рЌ > 7).
–озгл€немо г≥дрол≥з р≥зних солей.
1. —ол≥ сильноњ кислоти ≥ сильноњ основи г≥дрол≥зу не п≥дл€гають, рЌї 7 (NaCl; Ba(NO3)2; K2SO4).
2. —ол≥ сильноњ кислоти ≥ слабкоњ основи г≥дрол≥зуютьс€ з≥ зб≥льшенн€м концентрац≥њ Ќ+:
NH4NO3 + H2O ЂNH4OH + HNO3;
NH4+ + H2O ЂNH4OH + H+; pH < 7.
3. —ол≥ слабкоњ кислоти ≥ сильноњ основи г≥дрол≥зуютьс€ з≥ зб≥льшенн€м концентрац≥њ ќЌ-:
KCN + H2O ЂKOH + HCN;
CN- + H2O ЂOH- + HCN; pH > 7.
4. —ол≥ слабких кислот ≥ основ г≥дрол≥зуютьс€, ≥ рЌ розчину залежить в≥д њх в≥дносноњ сили ≥ взагал≥ близький до 7:
CH3COONH4 + H2O ЂNH4OH + CH3COOH;
CH3COO- + NH4+ + H2O ЂNH4OH + CH3COOH; pHї 7.
5. √≥дрол≥з солей багатоосновних кислот або багатокислотних основ в≥дбуваЇтьс€ ступ≥нчасто:
а) K3PO4 + H2O ЂK2HPO4 + KOH;
PO43- + H2O ЂHPO42- + OH-; pH > 7;
K2HPO4 + H2O ЂKH2PO4 + KOH;
HPO42- + H2O ЂH2PO4- + OH-; pH > 7,
г≥дрол≥з до к≥нц€ не проходить, тому що накопиченн€ ≥он≥в ќЌ- заважаЇ утворенню Ќ3–ќ4;
б) FeCl3 + H2O ЂFeOHCl2 + HCl;
Fe3+ + H2O ЂFe (OH)2+ + H+; pH < 7;
FeOHCl2 + H2O ЂFe(OH)2Cl + HCl;
Fe(OH)2+ + H2O ЂFe(OH)2+ + H+; pH < 7,
накопиченн€ ≥он≥в Ќ+ заважаЇ утворенню Fe(OH)3 по третьому ступеню;
в) г≥дрол≥з солей дуже слабких основ ≥ кислот (Al2S3, Cr2S3, Al2(CO3)3Е) в≥дбуваЇтьс€ до к≥нц€. ¬ розчин≥ так≥ сол≥ не ≥снують (в таблиц≥ розчинност≥ њх позначають знаком "-");
Cr2S3 + 6H2O = 2Cr(OH)3ѓ + 3H2S≠; pHї 7;

 Fe(CO3)3 + 6H2O = 2Fe(OH)3ѓ + 3H2CO3; pHї 7.
Fe(CO3)3 + 6H2O = 2Fe(OH)3ѓ + 3H2CO3; pHї 7.
3H2O 3CO2
≥льк≥сно г≥дрол≥з у розбавлених розчинах характеризуЇтьс€ константою г≥дрол≥зу. Ќаприклад, г≥дрол≥з NaCN:
NaCN + H2O ЂNaOH + HCN; CN-+ Ќ2ќ ЂHCN + OH-.
÷е Ц оборотний процес, тому йому в≥дпов≥даЇ константа р≥вноваги
=  .
.
ƒл€ розбавлених розчин≥в [H2O]>>[HCN], тод≥ можна вважати, що [H2O] = const,
|
|
|
K .[H2O]= K г =  =
=  =
= 
¬ цьому випадку константа г≥дрол≥зу г = Kw / K кис. ƒл€ сол≥ слабкоњ основи ≥ сильноњ кислоти K r = Kw / K осн, дл€ сол≥ слабкоњ основи ≥ кислоти K r = Kw /(K кис . K осн).
—туп≥нь г≥дрол≥зу bг - в≥дношенн€ числа г≥дрол≥зованих молекул до загального числа молекул сол≥ в розчин≥ Ц з⥈заний з константою г≥дрол≥зу сп≥вв≥дношенн€м br =  , де —ћ - мол€рна концентрац≥€ сол≥.
, де —ћ - мол€рна концентрац≥€ сол≥.
«г≥дно з принципом ле ЎательЇ розбавленн€ розчину сол≥ ≥ п≥двищенн€ температури посилюЇ г≥дрол≥з
ѕриклад 1. ¬изначити K r ≥ br дл€ 0,1ћ розчину Na3PO4 (константи дисоц≥ац≥њ H3PO4 в≥дпов≥дно дор≥внюють K 1 = 7.10-3; K 2 = 6.10-8; K 3 = 4.10-13).
–оз⥈зок:
1. г1 -? br1 -?
PO43- + H2O = HPO42-+ OH-. √≥дрол≥з по першому ступеню по⥈заний з дисоц≥ац≥Їю кислоти по третьому ступеню (HPO42- = H+ + PO43-) ≥ K 3. “ому
K r1 = Kw / K 3 = 10-14 / 4.10-13 = 0,025;
br1 = 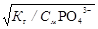 =
=  = 0,5 (50 %).
= 0,5 (50 %).
2. г2 -? bг2 -?
HPO42- + H2O = H2PO41- + OH-
K r2 = Kw / K 2 = 10-14 / 6.10-8 = 1,67.10-7;
br2 =  =
=  = 1,84.10-7 (0,184 %);
= 1,84.10-7 (0,184 %);
Cћ (HPO42-) = 0,1. 0,5, тому що br1 = 50 %, тобто 50 % Na3PO4 перетворилось у Na2HPO4.
¬≥дпов≥дь: Kr1 = 0,025, br1 = 50 %, Kr2 = 1,67.10-7, br2 = 0,184 %.






