ѕример 1. ѕользу€сь таблицей величин стандартных электродных потенциалов, приведите примеры нескольких окислителей, позвол€ющих перевести соединени€ Mn+2 в соединени€ Mn+7.
–ешение. ¬еличина электродного потенциала дл€ системы
MnO4Ц + 8H+ + 5ē = Mn2+ + 4H2O
равна 1,51 ¬. ѕоскольку φ0= ≈0окисл Ц ≈0восст, а критерием самопроизвольного протекани€ ќ¬– в стандартных услови€х €вл€етс€ φ0 > 0, то любое вещество Ц окислитель, стандартный электродный потенциал дл€ которого превышает 1,51 ¬, в принципе должен окисл€ть ион Mn2+ до иона ћnO4Ц
в кислой среде. “аковыми €вл€ютс€, например, ион —о3+, персульфаты, озон, фтор. Ёто подтверждаетс€ значени€ми величин электродных потенциалов:
††††††††††††††††††††††††††††† Co3+ + ē = Co2+;†††††††††††††††† E0 = 1,82 ¬;
†††††††††††††††††††††† S2O82Ц + 2ē = 2SO42Ц;†††††††† E0 = 2,01 ¬;
†††††††††††††††††††††† O3 + 2H+ +2ē = O2+H2O;†† E0 = 2,07 ¬;
†††††††††††††††††††††† F2 + 2H+ + 2ē =2HF;†††††††† E0 = 3,06 ¬.
ѕример 2. акие вещества (молекулы, ионы) не должны существовать в водном растворе?
–ешение. “аковыми должны быть соединени€, электродный потенциал которых дл€ соответствующей электрохимической пары превышает
потенциал разложени€ воды
O2 + 4H+ + 4ē = 2H2O;†††† E0 = 1,229 B.
¬ыпишем некоторые из них:
†††††††††††††††††† MnO4Ц + 8H+ + 5ē = Mn2+ + 4H2O;††††† E0 = 1,51 B;
††††††††††††† BrO3Ц + 6H+ + 5ē = 0,5Br2 + 3H2O;†††† E0 = 1,52 B;
††††††††††††† Cr2O72Ц + 14H+ + 6ē = 2Cr3+ + 7H2O; E0 = 1,33 B;
††††††††††††† Co3+ + ē = Co2+; ††††††††††††††††††††††††††††††††† E0 = 1,82 B;
††††††††††††† S2O82Ц + 2ē = 2SO42Ц;††††††††††††††††† ††† E0 = 2,01 B.
»з приведенных цифр следует, что ионы MnO4Ц, Cr2O72Ц, BrO3Ц, S2O82Ц €вл€ютс€ термодинамически неустойчивыми в водных растворах и должны окисл€ть воду. ќднако, кроме иона —о3+, остальные ионы кинетически устойчивы и реально существуют в водных растворах. —оединени€ же, дающие в водных растворах ион —о3+, окисл€ют воду.
ѕример 3. ќценить услови€, при которых серебро будет раствор€тьс€ в минеральных кислотах.
–ешение. ƒл€ того чтобы серебро раствор€лось в минеральных кислотах необходимо, чтобы процесс
2Ag + 2H+ = 2Ag+ + H2
был самопроизвольным, то есть имел DG< 0 или φ > 0. —огласно соотношени€м (1.27, 2.16):
Ag+ + ē =Ag; 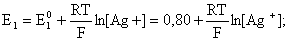
2H+ + 2ē = H2; 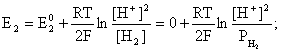
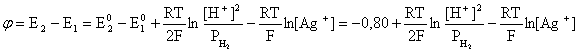
ѕроанализируем вопрос о том, какими должны быть величины [H+], 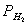 †и [Ag+] дл€ того, чтобы выполн€лось φ > 0. ѕусть [Ag+] = 1, [H+] = 1, получаем:
†и [Ag+] дл€ того, чтобы выполн€лось φ > 0. ѕусть [Ag+] = 1, [H+] = 1, получаем:
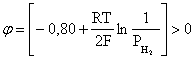 ;
;
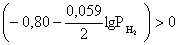 ;
;
lg 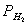 < Ц27,1;
< Ц27,1; 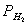 < 7,9×10Ц28 атм.
< 7,9×10Ц28 атм.
ѕусть [Ag+] = 1, 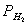 †= 1, получаем:
†= 1, получаем:
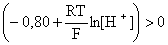 ;
;
0,059 lg [H+] > 0,80;
lg [H+] > 13,6;
[H+] > 4,0×1013 моль/л.
ак видно, дл€ осуществлени€ указанного процесса необходимо мизерное парциальное давление водорода в системе (глубочайшее разрежение!), либо неверо€тно больша€ кислотность раствора. » то, и другое практически реализовать невозможно. ѕусть 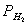 = 1, [Ќ+] = 1, получаем:
= 1, [Ќ+] = 1, получаем:
|
|
|
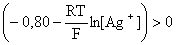 ;
;
0,059 lg [Ag+] < Ц 0,80;
lg [Ag+] < Ц 13,6;
[Ag+] < 2,5×10-14 моль/л.
“аким образом, если в растворе поддерживать равновесную концентрацию ионов серебра на уровне, меньшем 10-14 моль/л, то процесс растворени€ серебра в кислоте с [Ќ+] = 1 при 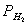 =1 станет самопроизвольным. ѕоддерживать столь малую концентрацию ионов серебра в растворе возможно только лишь в присутствии лигандов, образующих прочные комплексные соединени€ с ионами серебра. ‘актически в этом случае электродный потенциал EAg+/Agсмещаетс€ из области положительных значений к отрицательным величинам.
=1 станет самопроизвольным. ѕоддерживать столь малую концентрацию ионов серебра в растворе возможно только лишь в присутствии лигандов, образующих прочные комплексные соединени€ с ионами серебра. ‘актически в этом случае электродный потенциал EAg+/Agсмещаетс€ из области положительных значений к отрицательным величинам.
ѕример 4. Ќа примере комплексных соединений кобальта проанализировать возможность существовани€ ионов —о2+ и —о3+ в водном растворе.
–ешение. —тандартный электродный потенциал дл€ пары —о3+/—о2+ в водном растворе составл€ет 1,82 ¬, что значительно превышает потенциал разложени€ воды. »ными словами ион —о3+ (гидратированный!) не может существовать в водном растворе Ц он будет окисл€ть воду до ќ2 и восстанавливатьс€ до иона —о2+. — другой стороны, концентрационна€ зависимость этого потенциала выражаетс€ следующим выражением:
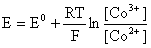 .
.
»з этого выражени€ видно, что чем меньше концентраци€ свободного (гидратированного!) иона —о3+ по сравнению с концентрацией иона —о2+ (гидратированного!), тем больше потенциал смещаетс€ в область отрицательных величин. “ака€ ситуаци€ возникает практически всегда, когда
в растворе наход€тс€ лиганды, образующие комплексы более прочные, чем аквакомплексы. “ак, в аммиачных растворах
[Co(NH3)6]3+ + ē = [Co(NH3)6]2+; E0=0,10 B.
¬ цианидных же растворах потенциал смещаетс€ настолько сильно в отрицательную область, что не только —о3+ не окисл€ет воду, а наоборот Ц комплексный ион [—o2+(CN)6]4Ц восстанавливает воду, окисл€€сь до [—o3+(CN)6]3Ц:
2 [—o2+(CN)6]4Ц + 2H2O = 2 [—o3+(CN)6]3Ц + H2 + 2OHЦ.
ѕример 5. Ќа основе значений следующих стандартных электродных потенциалов:
Tl+ + ē= Tl; E01 = Ц0,34 B;
Tl3+ + 2ē = Tl+; E02 = 1,25 B
определить величину стандартного электродного потенциала дл€ пары Tl3+/Tl.
–ешение. ”равнени€ окислительно-восстановительных полуреакций не €вл€ютс€ термохимическими и поэтому не позвол€ют выполн€ть над собой алгебраические операции. ¬ключим написанные две полуреакции и искомую
Tl3+ + 3ē = Tl; E0X
в реальные химические процессы, например, восстановлени€ иона Tl3+ водородом и взаимодействи€ талли€ с кислотой (протонами). ѕусть все участники нижеследующих процессов наход€тс€ в стандартных состо€ни€х. “огда имеем:
а) 2Tl + 2H+ = 2 Tl+ + H2††††††††††††††††††††††††††††† (2H+ + 2ē = H2, E0 = 0 B)
††† DG0a= Ц2F(0ЦE01) = 2FE01;
б) Tl3+ + H2 = Tl+ + 2H+
††† DG0б= Ц2F(E02 Ц 0) = Ц2FE02;
в) 2Tl3+ + 3H2 = 2 Tl + 6H+
††† DG0в= Ц6F(E0xЦ 0) = Ц6FE0x.
“ермохимическое уравнение "в" получаетс€ вычитанием уравнени€ "а" из удвоенного уравнени€ "б", таким образом:
DG0в =2DG0б †Ц DG0а;
Ц6FE0x = Ц4FE02 Ц 2FE01;
3E0x=2E02+E01;
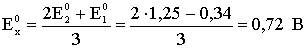 .
.
’»ћ»„≈— ќ≈ –ј¬Ќќ¬≈—»≈






