313. 2Co+3 + Tl+ = 2Co+2 + Tl+3,
а) фиксируем концентрацию [Co+3] = 0,3моль/л. ѕри увеличении [Tl+] в 2 раза, скорость реакции возрастает в 2 раза, т.е. пор€док по [Tl+] равен 1.
б) фиксируем [Tl+] = 0,1 моль/л. ѕри увеличении [Co+3] в 3 раза, скорость возрастает в 3 раза - пор€док по [Co+3] = 1. “.о. кинетическое уравнение имеет вид: V = K*[ Co+3]1 *[Tl+]1.
314. CH3CHO = CH4+CO. ѕри увеличении [CH3CHO] в 2 раза, скорость возрастает в 4 раза, т.е. пор€док равен 2. V=K* [CH3CHO]2.
315. 2A+3B=A2B3. а) фиксируем концентрацию [A] = 0,20 моль/л. ѕри увеличении [¬] в 4 раза, скорость реакции возрастает в 4 раза, т.е. пор€док по [¬] равен 1. б) фиксируем [¬] = 0,80 моль/л. »зменение [A] не приводит к изменению скорости. “.о. кинетическое уравнение имеет вид: V = K*[¬]1 . ѕосле увеличени€ давлени€ в 2 раза V/ = K*2[¬], скорость реакции возрастает в 2 раза: V/ / V = 2 раза.
316. 2NO+O2 = 2NO2, инетическое уравнение: V = K*[NO]2*[O2]1. 1.2*10-3 = *0.32 *0.15, следовательно, = 0,089 л2/моль2с.
317. H2+Y2 = 2HY, = 0,16, [H2] = 0,04, [I2] = 0,05 моль/л. Vисх. = K*[H2]*[I2] = 0,16*0,04*0,05 = 3,2*10-4. огда израсходовалась половина количества водорода, т.е. 0,02 моль/л, то израсходовалось столько же иода. ќсталось 0,02 моль/л водорода и 0,03 моль/л иода. V/ = 0,16*0,02*0,03 = 9,6*10-5.
318. 2NO+Cl2 = 2NOCl, [NOисх.] = 0,4 моль/л, [Cl2 исх.] = 0,3 моль/л. V = K*[NO]2 * [Cl2] = *0,42 *0,3 = 0,048 . ѕрореагировало 0,2 моль/л NO, следовательно, прореагировало 0,1 моль/л Cl2. ќсталось 0,2 моль/л NO и 0,2 моль/л Cl2. V = K * 0,22 * 0,2 = 0,008 . —корость уменьшилась в 0,048/0,008 = 6 раз.
319. —огласно правилу ¬ант-√оффа, при повышении температуры на 100 скорость реакции увеличиваетс€ в 2-4 раза. γ = 3, “1 = 800—, “2 = 1300—. V2/V1 = γ“2 -“1 / 10 = 3130-80 /10 = 243.
320. “емпературный коэффициент показывает, во сколько раз увеличиваетс€ скорость химической реакции при увеличении температуры на 10 градусов. γ = 3, ∆“ = 40, V2/V1 = γ“2 -“1 / 10 = 340 /10 = 81.
321. —огласно правилу ¬ант-√оффа, при повышении температуры на 100 скорость реакции увеличиваетс€ в 2-4 раза. γ = 1.8, V2/V1 = 50. ∆“ -?
V2/V1 = γ ∆“/10, 50 = 1.8 ∆“/10, ln50 = ∆“/10 *ln1.8, ∆“ = 66.50C.
322. ѕравило ¬ант-√оффа выведено не из фундаментальных законов, а опытным путем, поэтому оно называетс€ эмпирическим. ќно справедливо дл€ всех реакций, кроме ферментативных и реакций третьего пор€дка. “1 = 1000, “2 = 1500, 1 = 6*10-4, 2 = 7.2*10-2. 2 / 1 = γ ∆“ / 10 , 7.2*10-2 / 6*10-4 = γ (150-100)/10 , γ = 2.6.
323. “1=1200, “2=1700, 1=5,9*10-4, 2=6,7*10-2. 2 / 1 = γ ∆“ / 10 , 6,7*10-2 / 5,9*10-4 = γ 170-120/10 , γ = 2,6.
324. γ1 = 3, γ2 = 4, при температуре “1 : 1/ = 2/. ѕри температуре “2: 2// / 1// = 5. ∆“ -?
2 / 1 = γ ∆“ / 10 . 1// / 1/ = γ1 ∆“ / 10 (1) . 2// / 2/ = γ2 ∆“ / 10 (2). –азделим выражение (1) на выражение (2), получим: 1// / 2// = (γ1 ∆“ / 10) / (γ2 ∆“ / 10). 1/5 = (3 ∆“ / 10 ) / (4 ∆“ / 10). ln5 + (∆“ / 10) *ln3 = (∆“ / 10) * ln4, ∆“ = 560.
|
|
|
325. ”равнение јррениуса определ€ет зависимость константы скорости химической реакции от температуры. 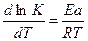 , = 0 * е -≈ а / RT .
, = 0 * е -≈ а / RT .
“1≠ = 288 , 1 = 3,1*10-4. “2 = 313 , 2 = 8,2*10-3.
ln(K2/K1) = Ea/R * (1/T1 - 1/T2).
ln(8,2*10-3 / 3,1*10-4) = ≈а / 8,31 * (1/288 - 1/313), следовательно, ≈а = 98,14 кƒж/моль.
326. ƒополнительна€ энерги€, необходима€ дл€ протекани€ между частицами химической реакции, называетс€ энергией активации. “1 = 673 , 1 = 2,2*10-4. “2 = 973 , 2 = 8,33. ln(K2/K1) = Ea/R * (1/T1 - 1/T2). ln(8,33 / 2,2*10-4) = ≈а / 8,31 * (1/673 - 1/973), ≈а = 191,4 кƒж/моль.
327. ≈а = 100 кƒж/моль “1 = 273 , 1 = 2,0*10-2. “2 = 373 , 2 =?. ln(K2/K1) = Ea/R * (1/T1 - 1/T2). ln( 2 / 2,0*10-2) = 100000 / 8,31 * (1/273 - 1/373), 2 = 2712.
328. а) 2NO = N2 + O2, ≈а = 290 кƒж/моль “1=300 , “2=310 , 2 / 1 =?. ln(K2/K1) = Ea/R * (1/T1 - 1/T2). ln( 2 / 1) = 290000 / 8,31 * (1/300 - 1/310), 2 / 1 = 43.
Ѕ) 2NO + O2 = 2Nќ2, ≈а = 10 кƒж/моль “1=300 , “2=310 , 2 / 1 =?. ln(K2/K1) = Ea/R * (1/T1 - 1/T2). ln( 2 / 1) = 10000 / 8,31 * (1/300 - 1/310), 2 / 1 = 1,14.
»зменение скоростей данных реакций не согласуетс€ с правилом ¬ант-√оффа.
329. ≈ј1 = 184 кƒж/моль, ≈ј2 = 107 кƒж/моль, пусть “=400 . 1 = 0 * е -≈ ј1 / RT , 2 = 0 * е -≈ ј2 / RT , 
 , 2 / 1 =1,15*1010 раз.
, 2 / 1 =1,15*1010 раз.
330.
| ѕроцесс | атализаторы |
| —интез аммиака из N2 и Ќ2 | Fe + K2O, Al2O3, MgO |
| —интез спиртов из —ќ и Ќ2 | ZnO-Cr2O3, CuO-ZnO- Cr2O3 |
| —интез углеводородов из —ќ и Ќ2 | Fe, Ni, Co+MgO |
| –иформинг | Pt/ Al2O3 |
| √идратаци€ олефинов | H3PO4 на носител€х |
| ѕроизводство серной кислоты | ќксид ванади€ (V) |
| ѕроизводство азотной кислоты | ѕлатинородиевые сетки |
| √идратаци€ этилена | H3PO4 и H2SO4 |
| ѕроизводство полиэтилена | Al(C2H5)3 и TiCl4 |
атализаторы используют дл€ увеличени€ скорости реакции, повышени€ ее селективности, конверсии и выхода целевого продукта.
≈ј1 = 200 кƒж/моль, ≈ј2 = 100 кƒж/моль, “=400 . 1 = 0 * е -≈ ј1 / RT , 2 = 0 * е -≈ ј2 / RT , 
 , 2 / 1 = 1,16*1013 раз.
, 2 / 1 = 1,16*1013 раз.
331. — точки зрени€ химической термодинамики, химическое равновесие характеризуетс€ посто€нством концентраций веществ во времени. —огласно химической кинетике, равновесие определ€етс€ равенством скоростей пр€мой и обратной реакций.
ј) N2 (г) + 3H2 (г) = 2NH3 (г), 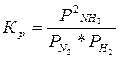
Ѕ) Fe3O4 + 4CO (г) = 3Fe (к) + 4CO2 (г), 
332. онстанта химического равновеси€ - это отношение произведени€ концентраций продуктов химической реакции к произведению концентраций исходных веществ, вз€тых в степен€х, равных их стехиометрическим коэффициентам.
ј) 4HCl + O2 (г)= 2Cl2(г) + 2H2O (г), 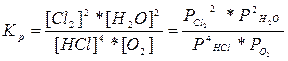
Ѕ) CaO (k) + H2O (г) = —a(OH)2 (к), 
333. —осто€ние реакции, при котором концентрации веществ не измен€ютс€ во времени, называетс€ равновесным. ќсновные признаки: если на реакцию, наход€щуюс€ в состо€нии химического равновеси€ оказать воздействие, то система перейдет в новое равновесное состо€ние, уменьшающее это воздействие. ≈сли воздействие сн€ть, то реакци€ вернетс€ к исходному состо€нию.
ј) CO(г) + H2O(г) = CO2(г) + H2(г) 
Ѕ) 2CuO(к) = 2Cu(к) + O2(г) 
334. ѕри истинном равновесии химическа€ реакци€ характеризуетс€ посто€нством концентраций всех веществ в течение сколь угодно долгого промежутка времени. ѕри этом реакци€ подчин€етс€ принципу Ће Ўателье. ѕри ложном химическом равновесии с течением времени концентрации веществ могут мен€тьс€, и реакци€ не подчин€етс€ принципу Ће Ўателье.
|
|
|
ј) 3O2(г) = 2ќ3 (г), 
Ѕ) Na2CO3 (к) + CO2(г) + H2O(г) = 2NaHCO3(к), 
335. «акон действующих масс: константа равновеси€ химической реакции равна отношению произведени€ концентраций продуктов реакции к произведению концентраций исходных веществ, вз€тых в степен€х, равных их стехиометрическим коэффициентам: ај + bB = cC + dD,  .
.
ј) N2 (г) + ќ2 (г) = 2Nќ(г),  ,
,
Ѕ) 3Fe(к) + 4H2O(г) = Fe3O4(к) + 4H2(г),  .
.
336. Ќа величину константы химического равновеси€ вли€ют: природа реагирующих веществ, температура, давление.
ј) Y2 (г) + H2 (г) = 2HY (г),  ,
,
Ѕ) MgO(к) + CO2(г) = MgCO3(к),  .
.
337. N2 (г) + 3H2 (г) = 2NH3 (г),
| Cисх. | х | у | |
| —равн. |
 , —исх. (N2) = [N2] + 0,5[NH3] = 3+ 0,5*4 = 5 моль/л.
, —исх. (N2) = [N2] + 0,5[NH3] = 3+ 0,5*4 = 5 моль/л.
—исх(Ќ2) = [Ќ2] + 1,5[NH3] = 9 + 1,5*4 = 15 моль/л.
338. CO + Cl2 = COCl2
| Cисх. | ’ | у | |
| —равн. | 0,2 | 0,3 | 1,2 |
 , —исх. (Cl2) = [Cl2] + [COCl2] = 0,3 + 1,2 = 1,5 моль/л.
, —исх. (Cl2) = [Cl2] + [COCl2] = 0,3 + 1,2 = 1,5 моль/л.
—исх(CO) = [CO] + [COCl2] = 0,2 + 1,2 = 1,4 моль/л.
339. CO + H2O = CO2 + H2
| Cисх. | 0,03 | 0,03 | ||
| —равн. | x | y | 0,01 | z |
—огласно уравнению реакции, водорода образуетс€ столько же, сколько и —ќ2: [CO2]=[H2]=0,01 моль/л. —ќ и Ќ2ќ израсходовалось 0,01 моль/л, следовательно [CO]=[H2O]=0,02 моль/л. 
 .
.
340. FeO(k) + CO = Fe(k) + CO2
| Cисх. | - | 0,05 | - | 0,01 |
| —равн. | - | 0,05-x | - | 0,01+x |
 0,01+x=0,025-0,5x, x=0,01. [CO] = 0,05 - 0,01 = 0,04 моль/л. [CO2] = 0,01 + 0,01 = 0,02 моль/л.
0,01+x=0,025-0,5x, x=0,01. [CO] = 0,05 - 0,01 = 0,04 моль/л. [CO2] = 0,01 + 0,01 = 0,02 моль/л.
341. Br2 (г) + H2 (г) = 2HBr (г),
| Cисх. | |||
| —равн. | 3-x | 1-x | 2x |
 , 3x2 + 4x - 3 = 0, x = 0,54.
, 3x2 + 4x - 3 = 0, x = 0,54.
[H2] = 3-0.54 = 2.46 моль/л, [Br2] = 1-0.54 = 0.46 моль/л, [HBr] = 2*0.54=1.08 моль/л.
w(H2)=(2.46/4)*100 %=61.5%, w(Br2)=(0.46/4)*100%=11.5%, w(HBr)=(1.08/4)*100%=27%.
342. N2O4 (г) = 2NO2 (г),
| Cисх. | 0.08 | |
| —равн. | 50% | X |
ѕрореагировало 0.08*0.5 = 0.04 моль/л N2O4. —ледовательно, образовалось 2*0.04 = 0.08 моль/л NO2 .
 .
.
343. Y2 (г) + H2 (г) = 2HY (г),
| Cисх. | 0.01 | 0.01 | |
| —равн. | 0.01-x | 0.01-x | 2x |
=40
 х = 0.0076, [HY]=2*0.0076=0.015моль/л.
х = 0.0076, [HY]=2*0.0076=0.015моль/л.
¬=(0.015*100)/(2*0.01)=76%
344. Y2 (г) + H2 (г) = 2HY (г),
| Cисх. | |||
| —равн. | 1-x | 2-x | 2x |
=50
 46х2 -150х + 100=0, х = 0.57, “еоретически может образоватьс€ 2 моль HY, образовалось 0.57*2 = 1.14 моль. ¬=(1.14*100%) / 2 = 57 %.
46х2 -150х + 100=0, х = 0.57, “еоретически может образоватьс€ 2 моль HY, образовалось 0.57*2 = 1.14 моль. ¬=(1.14*100%) / 2 = 57 %.
345. 2HY (г) = Y2 (г) + H2 (г)
| Cисх. | |||
| —равн. | 1-2x | x | x |
= 2*10-2
 х = 0.11, разложилось 2*0.11 = 0.22моль HY, степень разложени€ 22 %.
х = 0.11, разложилось 2*0.11 = 0.22моль HY, степень разложени€ 22 %.
346. Cl2 (г) = 2Cl(г)
| Cисх. | 0.04 | |
| —равн. | 0.04-x | 2x |
=4.2*10-4
 4х2 + 4.2*10-4х -1.68*10-5 = 0, х = 0.002, степень атомизации хлора равна (002/0.04)*100 % = 5 %.
4х2 + 4.2*10-4х -1.68*10-5 = 0, х = 0.002, степень атомизации хлора равна (002/0.04)*100 % = 5 %.
347. CO + H2O = CO2 + H2
| Cисх. | ||||
| —равн. | 1-x | 1-х | х | х |
=1. 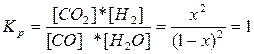 , х = 0.5, [CO2]=[H2]=0,5 моль/л, [CO]=[H2O]=0,5 моль/л.
, х = 0.5, [CO2]=[H2]=0,5 моль/л, [CO]=[H2O]=0,5 моль/л.
348. PCl5 = PCl3 + Cl2
| Cисх. | 0.3 | ||
| —равн. | 0.3-x | х | х |
=125.  , х2 + 125х - 37.5 = 0, х = 0.299. –азложилось PCl5: (0.299/0.3)*100% = 99.76 %.
, х2 + 125х - 37.5 = 0, х = 0.299. –азложилось PCl5: (0.299/0.3)*100% = 99.76 %.
349. ѕринцип Ће Ўателье: при оказании внешнего воздействи€ на реакцию, наход€щуюс€ в состо€нии равновеси€, ее равновесие смещаетс€ к состо€нию, уменьшающему это воздействие. ј) 2CO (г) + ќ2(г) = 2—ќ2 (г), ΔЌ0= -566 кƒж/моль. “.к. реакци€ экзотермическа€, то при понижении температуры ее равновесие сместитс€ вправо; т.к. количество газообразных продуктов реакции меньше количества газообразных исходных веществ, то при повышении давлени€ равновесие сместитс€ вправо. Ѕ) N2 (г) + O2 (г) = 2NO (г), ΔЌ0= 180 кƒж/моль. “.к. реакци€ эндотермическа€, то при понижении температуры ее равновесие сместитс€ влево; т.к. количество газообразных продуктов реакции равно количеству газообразных исходных веществ, то повышение давлени€ на равновесие не вли€ет.
350. ѕри оказании внешнего воздействи€ на систему, наход€щуюс€ в состо€нии равновеси€, положение равновеси€ смещаетс€ к состо€нию, уменьшающему это воздействие. ј) 2H2 (г) + O2 (г) = 2H2O (г), ΔЌ0 = - 483.6 кƒж/моль. “.к. реакци€ экзотермическа€, то при повышении температуры ее равновесие сместитс€ влево; т.к. количество газообразных продуктов реакции меньше количества газообразных исходных веществ, то при понижении давлени€ равновесие сместитс€ вправо. Ѕ) CaCO3 (k) = CaO (k) + CO2(г), ΔЌ0 = 179,0 кƒж/моль. “.к. реакци€ эндотермическа€, то при повышении температуры ее равновесие сместитс€ вправо; т.к. газообразные вещества - продукты реакции CO2 , то понижение давлени€ сместит равновесие вправо.
|
|
|
351. N2 (г) + 3H2 (г) = 2NH3 (г), ΔH0= -92.4 кƒж/моль. а) реакци€ экзотермическа€, поэтому при увеличении температуры равновесие сместитс€ влево, а при понижении - вправо. Ѕ) “.к. количество газообразных продуктов реакции меньше количества газообразных исходных веществ, то при повышении давлени€ равновесие сместитс€ вправо, а при понижении давлени€ - влево. ¬) равновесие сместитс€ вправо при повышении концентрации азота и водорода и понижении концентрации аммиака, при повышении концентрации аммиака и понижении концентрации азота и водорода равновесии сместитс€ влево. √) введение промоторов не смещает положение равновеси€. д) ѕоглощение аммиака сместит равновесие вправо, а поглощение азота и водорода - влево. ≈) введение инертного газа аналогично повышению давлени€.
352. 4HCl(г) + O2(г) = 2Cl2(г) + 2H2O(г), ΔH0= -116.4 кƒж/моль. а) равновесие сместитс€ в сторону исходных веществ при повышении температуры, уменьшении давлени€, увеличении концентрации хлора и воды, понижении концентрации кислорода и сол€ной кислоты. Ѕ) равновесие сместитс€ в сторону продуктов при понижении температуры, увеличении давлени€, уменьшении концентрации хлора и воды, увеличении концентрации кислорода и сол€ной кислоты.
353. 3Fe(к) + 4H2O(г) = Fe3O4(к) + 4H2(г), ΔH0= -49,9 кƒж/моль. ƒл€ увеличени€ выхода водорода необходимо понизить температуру, т. к. реакци€ протекает с выделением тепла. ƒавление не вли€ет на положение равновеси€, т.к. количество газообразных продуктов равно количеству газообразных исходных веществ.
354. MgO(k) + CO2 (г) = MgCO3(к), ΔH0= -111,7 кƒж/моль. ѕоглощение CO2 будет происходить при понижении температуры и повышении давлени€. ¬осстановление поглотител€ будет протекать при повышении температуры и понижении давлени€.
355. CaO + H2O = Ca(OH)2.
ΔH0 = -986.6+635.5+241.8 = -109.3 кƒж/моль. ΔS0 = 76.1-39.7-188.7 = -152.3 ƒж/(K*моль)
ΔG0 = ΔH0 - “ * ΔS0. ΔG0 = -R*T*lnKp => Kp = e-ΔG/RT
а) “ = 300 . ΔG0 = -109.3 + 300*152.3*10-3 = -63.61 кƒж/моль. р = 1.2*1011
б) “ = 1000 . ΔG0 = -109.3 + 1000*152.3*10-3 = 43 кƒж/моль. р = 5.7*10-3
–асчет согласуетс€ с принципом Ће-Ўателье, т.к. при повышении температуры равновесие экзотермической реакции смещаетс€ влево.
356. N2O3 = NO + NO2.
ΔH0 = 90.2 + 33.5 - 83.3 = 40.4 кƒж/моль. ΔS0 = 210.6 + 240.2 - 178.2 = 272.6 ƒж/(K*моль)
ΔG0 = ΔH0 - “ * ΔS0. ΔG0 = -R*T*lnKp => Kp = e-ΔG/RT
а) “ = 00 C = 273 . ΔG0 = 40.4 - 273*272.6*10-3 = -34.0 кƒж/моль. р = 3.3*106
б) “ = 373 . ΔG0 = 40.4 - 373*272.6*10-3 = -61.3 кƒж/моль. р = 3.9*108
–асчет согласуетс€ с принципом Ће-Ўателье, т.к. при повышении температуры равновесие эндотермической реакции смещаетс€ вправо.
357. 2H2O = 2H2 + ќ2.
ΔH0 = 2*241.8 = 483.6 кƒж/моль. ΔS0 = 2*130.5 - 205 - 2*188.7 = -321.4 ƒж/(K*моль)
ΔG0 = ΔH0 - “ * ΔS0. ΔG0 = -R*T*lnKp => Kp = e-ΔG/RT
а) “ = 500 . ΔG0 = 483.6 + 500*321.4*10-3 = 644.3 кƒж/моль. р = 4.5*10-68
|
|
|
б) “ = 1000 . ΔG0 = 483.6 + 1000*321.4*10-3 = 805 кƒж/моль. р = 8.5*10-43
–асчет согласуетс€ с принципом Ће-Ўателье, т.к. при повышении температуры равновесие эндотермической реакции смещаетс€ вправо.
358. ZnO + H2 = Zn + H2O.
ΔH0 = -241.8 + 350.6 = 108.8 кƒж/моль. ΔS0 = 41.6 + 188.7 - 43.6 - 130.5 = 56.2 ƒж/K*моль
ΔG0 = ΔH0 - “ * ΔS0. ΔG0 = -R*T*lnKp => Kp = e-ΔG/RT
а) “ = 300 . ΔG0 = 108.8 - 300*56.2*10-3 = 91.94 кƒж/моль. р = 9.6*10-17
б) “ = 1000 . ΔG0 = 108.8 - 1000*56.2*10-3 = 52.6 кƒж/моль. р = 5.7*10-3
–асчет согласуетс€ с принципом Ће-Ўателье, т.к. при повышении температуры равновесие эндотермической реакции смещаетс€ вправо.
359. FeO + H2 = Fe +H2ќ.
ΔH0 = -241.8 + 264.8 = 23 кƒж/моль. ΔS0 = 27.3 + 188.7 - 60.8 -130.5 = 24.7 ƒж/(K*моль)
ΔG0 = ΔH0 - “ * ΔS0. ΔG0 = -R*T*lnKp => Kp = e-ΔG/RT
а) “ = 500 . ΔG0 = 23 - 500*24.7*10-3 = 10.65 кƒж/моль. р = 0.077
б) “ = 1000 . ΔG0 = 23 - 1000*24.7*10-3 = -1.7 кƒж/моль. р = 1.23
–асчет согласуетс€ с принципом Ће-Ўателье, т.к. при повышении температуры равновесие эндотермической реакции смещаетс€ вправо.
360. 2HI = H2 + I2.
ΔH0 = 2*26.6 = 53.2 кƒж/моль. ΔS0 = 130.5 + 116.2 - 2*206.5 = -166.3 ƒж/(K*моль)
ΔG0 = ΔH0 - “ * ΔS0.
“ = 400 . ΔG0 = 53.2 + 400*166.3*10-3 = 119.7 кƒж/моль.
ΔG0 = -R*T*lnKp => Kp = e-ΔG/RT => р = 2.2*10-16
—огласно закону ќствальда р =  . “.к. константа равновеси€ очень мала, то можно пренебречь знаменателем. “огда
. “.к. константа равновеси€ очень мала, то можно пренебречь знаменателем. “огда  = 4.8*10-5 %. данному расчету принцип Ће-Ўателье применить нельз€, т.к. не рассматриваетс€ смещение равновеси€.
= 4.8*10-5 %. данному расчету принцип Ће-Ўателье применить нельз€, т.к. не рассматриваетс€ смещение равновеси€.
361. ¬ состав атома вход€т протоны и нейтроны (они образуют €дро атома) и электроны. ѕротоны €вл€ютс€ носител€ми положительного зар€да, их количество определ€ет величину зар€да €дра и атомный номер химического элемента, масса поко€ протона: 1,673*10-27 кг, зар€д: 1,602*10-19 л. Ёлектроны - носители отрицательного зар€да, масса поко€ электрона: 9,109*10-31 кг, зар€д: 1,602*10-19 л. Ќейтроны - нейтральные частицы (зар€д равен нулю), масса поко€: 1,675*10-27 кг.
362.
| „астица | символ | ћасса поко€, кг | ќтносительна€ масса, а.е.м. | «ар€д, л |
| протон | p | 1,673*10-27 | 1,007276 | 1,602*10-19 |
| нейтрон | n | 1,675*10-27 | 1,008665 | |
| электрон | e | 9,109*10-31 | 0,000549 | 1,602*10-19 |
363. —огласно модели –езерфорда, атом состоит из €дра, в котором сосредоточена основна€ масса атома, и электронов, движущихс€ на относительно большом рассто€нии от €дра. ќднако эта модель противоречила факту устойчивого существовани€ атомов: в результате движени€ электроны расходуют энергию прит€жени€ с €дром, и через 10-8 секунды они должны упасть на €дро. роме того, электроны тер€ют энергию за счет излучени€, образующего сплошной спектр, что также противоречило фактам: все атомные спектры имеют линейчатый характер.






