121. Ёквивалент - реальна€ или условна€ частица вещества, котора€ может замещать, присоедин€ть, высвобождать или быть каким-либо эквивалентна одному иону водорода в кислотно-основных или ионообменных реакци€х или одному электрону в окислительно-восстановительных реакци€х. Ёквивалентна€ масса - масса одного эквивалента вещества. ћол€рна€ масса эквивалента - масса одного моль эквивалентов. Ёквивалентна€ масса (Mэ), валентность (¬), атомна€ масса (ј) элемента св€заны соотношением: 
Ќапример, валентность азота в аммиаке равна 3, следовательно, мол€рна€ масса эквивалента азота: ћэ(N) = 14/3 = 4,66 г/моль
122.  . Ё(Li) в LiH=1, ћэ(Li)=39/1=39 г/моль; Ё(Be) в BeH2 = 2 ћэ(Be)=9/2=4.5 г/моль; Ё(¬) в ¬Ќ3=3 ћэ(¬)=11/3=3.66 г/моль; Ё(—) в —Ќ4=4 ћэ(—)=12/4=3 г/моль.
. Ё(Li) в LiH=1, ћэ(Li)=39/1=39 г/моль; Ё(Be) в BeH2 = 2 ћэ(Be)=9/2=4.5 г/моль; Ё(¬) в ¬Ќ3=3 ћэ(¬)=11/3=3.66 г/моль; Ё(—) в —Ќ4=4 ћэ(—)=12/4=3 г/моль.
123.
| ¬-во | Ёквивалент Cr | Ёквивалентна€ масса Cr | ћол€рна€ масса экв-та Cr |
| CrO | 52/2=26 | 52/2=26 г/моль | |
| Cr2O3 | 52/3=17.3 | 52/3=17.3 г/моль | |
| CrO3 | 52/6=8.67 | 52/6=8.67 г/моль |
124.
| ¬ещество | Ёквивалент V | Ёквивалентна€ масса V | ћол€рна€ масса экв-та V |
| VO | 51/2=25.5 | 51/2=25.5 г/моль | |
| V 2O3 | 51/3=17 | 51/3=17 г/моль | |
| VO2 | 51/4=12.75 | 51/4=12.75 г/моль | |
| V2O5 | 51/5=10.2 | 51/5=10.2 г/моль |
125. «акон эквивалентов: дл€ молекул€рных соединений массовые количества составл€ющих элементов пропорциональны их химическим эквивалентам; при отсутствии молекул€рной структуры массовые количества составл€ющих элементов могут отклон€тьс€ от значений их химических эквивалентов:  . m(Me)=16.74 г., m(MeO)=21.54 г., ћэ(ћеќ)=ћэ(ће)+ћэ(ќ)=ћэ(ће)+8.
. m(Me)=16.74 г., m(MeO)=21.54 г., ћэ(ћеќ)=ћэ(ће)+ћэ(ќ)=ћэ(ће)+8.  , следовательно, ћэ(ће)=28 г/моль. “.к. валентность=2, то атомна€ масса металла=28*2=56 - железо.
, следовательно, ћэ(ће)=28 г/моль. “.к. валентность=2, то атомна€ масса металла=28*2=56 - железо.
126.  m(Sn)=0.92г., m(SnO)=1.17г., ћэ(Snќ)=ћэ(Sn)+ћэ(ќ)=ћэ(Sn)+8.
m(Sn)=0.92г., m(SnO)=1.17г., ћэ(Snќ)=ћэ(Sn)+ћэ(ќ)=ћэ(Sn)+8.  , следовательно, ћэ(Sn)=29.7 г/моль. “.к. атомна€ масса олова=118.6, то валентность олова ¬=ј/ћэ=118.6/29.7=4, следовательно, это SnO2.
, следовательно, ћэ(Sn)=29.7 г/моль. “.к. атомна€ масса олова=118.6, то валентность олова ¬=ј/ћэ=118.6/29.7=4, следовательно, это SnO2.
127. w%(O)=40.05%. ¬озьмем 100 грамм оксида, масса кислорода равна m(ќ)=40.05 грамм, масса металла m(ће)=100-40.05=59.95г.  , следовательно,
, следовательно,  , ћэ(ће)=12 г/моль. “.к. валентность=4, то атомна€ масса металла=12*4=48 - титан TiO2.
, ћэ(ће)=12 г/моль. “.к. валентность=4, то атомна€ масса металла=12*4=48 - титан TiO2.
128. ќбъем кислорода равен V(ќ)=6.72 литра, масса металла m(ће)=10.8г.  , следовательно,
, следовательно,  , ћэ(ће)=9 г/моль. “.к. валентность=3, то атомна€ масса металла=9*3=27 - алюминий.
, ћэ(ће)=9 г/моль. “.к. валентность=3, то атомна€ масса металла=9*3=27 - алюминий.
129.  , следовательно,
, следовательно,  , ћэ(ћеќ) = 39.75 г/моль, ћэ(ће) = ћэ(ћеќ)-ћэ(ќ)=39.75-8=31.75 г/моль. “.к. валентность=2, то атомна€ масса металла = 31.75*2 = 63.5 - медь. CuO.
, ћэ(ћеќ) = 39.75 г/моль, ћэ(ће) = ћэ(ћеќ)-ћэ(ќ)=39.75-8=31.75 г/моль. “.к. валентность=2, то атомна€ масса металла = 31.75*2 = 63.5 - медь. CuO.
130. w%(Ќ) = 4.76%. ¬озьмем 100 грамм гидрида, масса водорода равна m(Ќ) = 4.76 г., масса металла m(ће) = 100-4.76 = 95.24г.  , следовательно,
, следовательно, 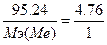 , ћэ(ће) = 20 г/моль. “.к. ¬ = 2, то атомна€ масса металла = 20*2 = 40 - кальций —аH2.
, ћэ(ће) = 20 г/моль. “.к. ¬ = 2, то атомна€ масса металла = 20*2 = 40 - кальций —аH2.
131. а) w%(O)=11.18%. ¬озьмем 100 грамм оксида, масса кислорода равна m(ќ)=11.18 грамм, масса меди m(Cu)=100-11.18=88.82г.  , следовательно,
, следовательно,  , ћэ(Cu)=63.5 г/моль. “.к. атомна€ масса меди=63.5, то валентность=63.5/63.5=1 - Cu2O.
, ћэ(Cu)=63.5 г/моль. “.к. атомна€ масса меди=63.5, то валентность=63.5/63.5=1 - Cu2O.
|
|
|
б) w%(O)=20.11%. ¬озьмем 100 грамм оксида, масса кислорода равна m(ќ)=20.11 грамм, масса меди m(Cu)=100-20.11=79.89г.  , следовательно,
, следовательно,  , ћэ(Cu)=31.6 г/моль. “.к. атомна€ масса меди=63.5, то валентность=63.5/31.6=2 - CuO.
, ћэ(Cu)=31.6 г/моль. “.к. атомна€ масса меди=63.5, то валентность=63.5/31.6=2 - CuO.
132. а) w%(O)=7.17%. ¬озьмем 100 грамм оксида, масса кислорода равна m(ќ)=7.17 грамм, m(Pb)=100-7.17=92.83г.  , следовательно,
, следовательно, 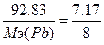 , ћэ(Pb)=103.6 г/моль. “.к. атомна€ масса свинца=206.7, то валентность=206.7/103.6=2 - PbO.
, ћэ(Pb)=103.6 г/моль. “.к. атомна€ масса свинца=206.7, то валентность=206.7/103.6=2 - PbO.
б) w%(O)=13.38%. ¬озьмем 100 грамм оксида, масса кислорода равна m(ќ)=13.38 грамм, m(Pb)=100-13.38=86.62г.  , следовательно,
, следовательно,  , ћэ(Pb)=51.8 г/моль. “.к. атомна€ масса свинца=206.7, то валентность=206.7/51.8=4 - PbO2.
, ћэ(Pb)=51.8 г/моль. “.к. атомна€ масса свинца=206.7, то валентность=206.7/51.8=4 - PbO2.
133. а) w%(F)=34.89%. ¬озьмем 100 грамм вещества, масса фтора равна m(F)=34.89 грамм, m(Cl)=100-34.89=65.11г.  , следовательно,
, следовательно,  , ћэ(Cl)=35.5 г/моль. “.к. атомна€ масса хлора=35.5, то валентность=35.5/35.5=1 - ClF.
, ћэ(Cl)=35.5 г/моль. “.к. атомна€ масса хлора=35.5, то валентность=35.5/35.5=1 - ClF.
б) w%(F)=61.65%. ¬озьмем 100 грамм вещества, масса фтора равна m(F)=61.65 грамм, m(Cl)=100-61.65=38.35г.  , следовательно,
, следовательно,  , ћэ(Cl)=11.8 г/моль. “.к. атомна€ масса хлора=35.5, то валентность=35.5/11.8=3 - ClF3.
, ћэ(Cl)=11.8 г/моль. “.к. атомна€ масса хлора=35.5, то валентность=35.5/11.8=3 - ClF3.
в) w%(F)=72.82%. ¬озьмем 100 грамм вещества, масса фтора равна m(F)=72.82 грамм, m(Cl)=100-72.82=27.18г.  , следовательно,
, следовательно,  , ћэ(Cl)=7.1 г/моль. “.к. атомна€ масса хлора=35.5, то валентность=35.5/7.1=5 - ClF5
, ћэ(Cl)=7.1 г/моль. “.к. атомна€ масса хлора=35.5, то валентность=35.5/7.1=5 - ClF5
г) w%(F)=78.96%. ¬озьмем 100 грамм вещества, масса фтора равна m(F)=78.96 грамм, m(Cl)=100-78.96=21.04г.  , следовательно,
, следовательно,  , ћэ(Cl)=5.1 г/моль. “.к. атомна€ масса хлора=35.5, то валентность=35.5/5.1=7 - ClF7.
, ћэ(Cl)=5.1 г/моль. “.к. атомна€ масса хлора=35.5, то валентность=35.5/5.1=7 - ClF7.
134. а) w%(S)=39.1%. ¬озьмем 100 грамм вещества, масса серы равна m(S)=39.1 грамм, ¬(S)=2, следовательно, ћэ(S)=32/2=16 г/моль. m(As)=100-39.1=60.9г.  , следовательно,
, следовательно,  , ћэ(As)=24.9 г/моль. “.к. атомна€ масса мышь€ка = 75, то валентность=75/24.9=3 - As2S3.
, ћэ(As)=24.9 г/моль. “.к. атомна€ масса мышь€ка = 75, то валентность=75/24.9=3 - As2S3.
б) w%(S)=51.7%. ¬озьмем 100 грамм вещества, масса серы равна m(S)=51.7 грамм. ¬(S)=2, следовательно, ћэ(S)=32/2=16 г/моль. m(As)=100-51.7=48.3г.  , следовательно,
, следовательно,  , ћэ(As)=15 г/моль. “.к. атомна€ масса мышь€ка = 75, то валентность=75/15=5 - As2S5.
, ћэ(As)=15 г/моль. “.к. атомна€ масса мышь€ка = 75, то валентность=75/15=5 - As2S5.
135. m(√)=1.591г., V6(ќ)=70.2 мл. »звестно, что Vэ(ќ)=5.6 л. ѕо закону эквивалентов:  =>
=>  => ћэ(√)=127 г/моль. ј(√)=127 а.е.м. Ёто иод.
=> ћэ(√)=127 г/моль. ј(√)=127 а.е.м. Ёто иод.
136. m(Me) = 1.2 г., m(MeO) = 2 г., m(MeS) = 2.8 г.  . ћэ(ћеќ) = ћэ(ће) + ћэ(ќ) = ћэ(ће) + 8.
. ћэ(ћеќ) = ћэ(ће) + ћэ(ќ) = ћэ(ће) + 8.  , следовательно, ћэ(ће) = 12 г/моль. ћэ(ћеS) = ћэ(Me) + ћэ(S) = ћэ(S) + 12.
, следовательно, ћэ(ће) = 12 г/моль. ћэ(ћеS) = ћэ(Me) + ћэ(S) = ћэ(S) + 12. 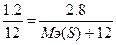 => Mэ(S) = 16 г/моль.
=> Mэ(S) = 16 г/моль.
137. —уд=0.218 ƒж/(г. ). ћэ(ће)=29.65 г/моль. «акон ƒюлонга-ѕти: ј(ће)*—уд≈26. ј(ће) ≈ 26 / 0.218 = 119.3 г/моль. ¬алентность металла: ¬(ће) = ј/ћэ = 119.3/29.65 = 4. “очна€ атомна€ масса равна ј(ће) = ¬*ћэ = 4*29.65 = 118.6 г/моль.
138. —уд=0.454 ƒж/(г. ). w%(Me)=70.97%. ¬озьмем 100 грамм оксида. m(Me)=70.97 г., m(O)=100-70.97=29.03 г.  . ћэ(ћеќ) = ћэ(ће) + ћэ(ќ) = ћэ(ће) + 8.
. ћэ(ћеќ) = ћэ(ће) + ћэ(ќ) = ћэ(ће) + 8.  , следовательно, ћэ(ће)=29.35 г/моль. «акон ƒюлонга-ѕти: ј(ће)*—уд≈26. ј(ће) ≈ 26 / 0.454 = 57.2 г/моль. ¬алентность металла: ¬(ће) = ј/ћэ = 57.2/29.35 = 2. “очна€ атомна€ масса равна ј(ће) = ¬*ћэ = 2*29.35 = 58.7 г/моль. - Ni.
, следовательно, ћэ(ће)=29.35 г/моль. «акон ƒюлонга-ѕти: ј(ће)*—уд≈26. ј(ће) ≈ 26 / 0.454 = 57.2 г/моль. ¬алентность металла: ¬(ће) = ј/ћэ = 57.2/29.35 = 2. “очна€ атомна€ масса равна ј(ће) = ¬*ћэ = 2*29.35 = 58.7 г/моль. - Ni.
139. —уд=0.281 ƒж/(г. ). m(Me)=0.6 г., m(ћеO)=0.9 г.  . ћэ(ћеќ) = ћэ(ће) + ћэ(ќ) = ћэ(ће) + 8.
. ћэ(ћеќ) = ћэ(ће) + ћэ(ќ) = ћэ(ће) + 8.  , следовательно, ћэ(ће)=16 г/моль. «акон ƒюлонга-ѕти: ј(ће)*—уд≈26. ј(ће) ≈ 26 / 0.281 = 92.53 г/моль. ¬алентность металла: ¬(ће) = ј/ћэ = 92.53/16 = 6. “очна€ атомна€ масса равна ј(ће) = ¬*ћэ = 6*16 = 96 г/моль. - молибден. ѕогрешность=
, следовательно, ћэ(ће)=16 г/моль. «акон ƒюлонга-ѕти: ј(ће)*—уд≈26. ј(ће) ≈ 26 / 0.281 = 92.53 г/моль. ¬алентность металла: ¬(ће) = ј/ћэ = 92.53/16 = 6. “очна€ атомна€ масса равна ј(ће) = ¬*ћэ = 6*16 = 96 г/моль. - молибден. ѕогрешность= 
140. —уд=0.147 ƒж/(г. ). m(Me)=0.2698 г., m(ћеO)=0.3402 г.  . ћэ(ћеќ) = ћэ(ће) + ћэ(ќ) = ћэ(ће) + 8.
. ћэ(ћеќ) = ћэ(ће) + ћэ(ќ) = ћэ(ће) + 8.  , следовательно, ћэ(ће)=30.66 г/моль. «акон ƒюлонга-ѕти: ј(ће)*—уд≈26. ј(ће) ≈ 26 / 0.147 = 176.87 г/моль. ¬алентность металла: ¬(ће) = ј/ћэ = 176.87/30.66 = 6. “очна€ атомна€ масса равна ј(ће) = ¬*ћэ = 6*30.66 = 183.96 г/моль. - вольфрам.
, следовательно, ћэ(ће)=30.66 г/моль. «акон ƒюлонга-ѕти: ј(ће)*—уд≈26. ј(ће) ≈ 26 / 0.147 = 176.87 г/моль. ¬алентность металла: ¬(ће) = ј/ћэ = 176.87/30.66 = 6. “очна€ атомна€ масса равна ј(ће) = ¬*ћэ = 6*30.66 = 183.96 г/моль. - вольфрам.
|
|
|
141. m(Me)=40 г., V(Ќ2)=14.6 л. T=18 oC=291 K, P=1.013*105 ѕа, —уд=0.39 ƒж/(г. ). Ќайдем V(H2) при н. у.:  =>
=>  литров. «акон эквивалентов:
литров. «акон эквивалентов:  =>
=>  , ћэ(ће)=32.71 г/моль. «акон ƒюлонга-ѕти: ј(ће)*—уд≈26. ј(ће) ≈ 26 / 0.39 = 66.67 г/моль. ¬алентность металла: ¬(ће) = ј/ћэ = 66.67/31.41 = 2. “очна€ атомна€ масса равна ј(ће) = ¬*ћэ = 2*32.71 = 65.42 г/моль. - цинк.
, ћэ(ће)=32.71 г/моль. «акон ƒюлонга-ѕти: ј(ће)*—уд≈26. ј(ће) ≈ 26 / 0.39 = 66.67 г/моль. ¬алентность металла: ¬(ће) = ј/ћэ = 66.67/31.41 = 2. “очна€ атомна€ масса равна ј(ће) = ¬*ћэ = 2*32.71 = 65.42 г/моль. - цинк.
142. m(Me)=2.162 г., V(Ќ2)=14.78 л. T=327 oC=600 K, P=101.325*102 кѕа, —уд=2.209 ƒж/(г. ).  , следовательно,
, следовательно,  моль. «акон эквивалентов:
моль. «акон эквивалентов:  =>
=>  , ћэ(ће)=12 г/моль. «акон ƒюлонга-ѕти: ј(ће)*—уд≈26. ј(ће) ≈ 26 / 2.209 = 11.77 г/моль. ¬алентность металла: ¬(ће) = ј/ћэ = 11.77/12 = 1. “очна€ атомна€ масса равна ј(ће) = ¬*ћэ = 1*12 = 12 г/моль. - углерод.
, ћэ(ће)=12 г/моль. «акон ƒюлонга-ѕти: ј(ће)*—уд≈26. ј(ће) ≈ 26 / 2.209 = 11.77 г/моль. ¬алентность металла: ¬(ће) = ј/ћэ = 11.77/12 = 1. “очна€ атомна€ масса равна ј(ће) = ¬*ћэ = 1*12 = 12 г/моль. - углерод.
143. Ёквивалентна€ масса кислоты равна отношению мол€рной массы кислоты к количеству замещаемых протонов: 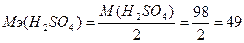 г/моль.
г/моль.
Ёквивалентна€ масса основани€ равна отношению мол€рной массы основани€ к количеству замещаемых ќЌ-групп: 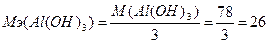 г/моль.
г/моль.
Ёквивалентна€ масса соли равна отношению мол€рной массы соли к произведению количества катионов на их валентность:  г/моль.
г/моль.
144. а) Ќ3–ќ4 + ќЌ = Ќ2–ќ4 + Ќ2ќ ћэ(Ќ3–ќ4)=ћ(Ќ3–ќ4)/Ё=98/1=98 г/моль.
б) Ќ3–ќ4 + 2 ќЌ = 2Ќ–ќ4 + 2Ќ2ќ ћэ(Ќ3–ќ4)=ћ(Ќ3–ќ4)/Ё=98/2=49 г/моль.
в) Ќ3–ќ4 + 3 ќЌ = Ќ2–ќ4 + Ќ2ќ ћэ(Ќ3–ќ4)=ћ(Ќ3–ќ4)/Ё=98/3=32.67 г/моль.
145. a) Fe(OH)3 + HCl = Fe(OH)2Cl + H2O ћэ(Fe(OH)3)=ћ(Fe(OH)3)/Ё=107/1=107 г/моль.
б) Fe(OH)3 + 2HCl = Fe(OH)Cl2 + 2H2O ћэ(Fe(OH)3)=ћ(Fe(OH)3)/Ё=107/2=53.5 г/моль.
в) Fe(OH)3 + 3HCl = Fe(OH)Cl3 + 3H2O ћэ(Fe(OH)3)=ћ(Fe(OH)3)/Ё=107/3=35.67 г/моль.
146. a) NaHCO3 + NaOH = Na2CO3 + H2O ћэ(NaHCO3) = ћ(NaHCO3)/Ё = 84/1 = 84 г/моль.
б) NaHCO3 + BaCl2 = BaCO3 + NaCl + HCl ћэ(NaHCO3) = ћ(NaHCO3)/Ё = 84/2 = 42 г/моль.
147. m(щелочи) = 1.00 г, m(HCl) = 2.14 г. ћэ(щелочи) =?. «акон эквивалентов:  , следовательно, ћэ(щелочи) = m(щелочи)*ћэ(HCl)/m(HCl) = 1*36.5/2.14 = 17 г/моль.
, следовательно, ћэ(щелочи) = m(щелочи)*ћэ(HCl)/m(HCl) = 1*36.5/2.14 = 17 г/моль.
148. m(кислоты) = 0.18 г, m(NaOH) = 0.1 г. ћэ(кислоты) =?. «акон эквивалентов:  , следовательно, ћэ(кислоты) = m(кислоты)*ћэ(NaOH)/m(NaOH) = 0.18*40/0.1 = 72 г/моль.
, следовательно, ћэ(кислоты) = m(кислоты)*ћэ(NaOH)/m(NaOH) = 0.18*40/0.1 = 72 г/моль.
149. m( ќЌ)=14 г, m(H2SO4) = 24.5 г. ћэ(H2SO4)=?. «акон эквивалентов:  , следовательно, ћэ(H2SO4) = m(H2SO4)*ћэ( ќЌ)/m( ќЌ)=24.5*56/14=98 г/моль. Ёквивалент H2SO4 равен 1, следовательно, образовалась однозамещенна€ соль (кисла€) ЌSO4
, следовательно, ћэ(H2SO4) = m(H2SO4)*ћэ( ќЌ)/m( ќЌ)=24.5*56/14=98 г/моль. Ёквивалент H2SO4 равен 1, следовательно, образовалась однозамещенна€ соль (кисла€) ЌSO4
150. m( ќЌ)=1.291 г., m(H3PO3)=0.943 г. ћэ(H3PO3)=?. «акон эквивалентов:  , следовательно, ћэ(H3PO3) = m(H3PO3)*ћэ( ќЌ)/m( ќЌ)=0.943*56/1.291=41 г/моль. ќсновность H3PO3 равна ћ(H3PO3)/ћэ(H3PO3)=82/41=2, следовательно, образовалась двузамещенна€ соль 2Ќ–O3, данна€ кислота двухосновна€, так как один атом водорода св€зан непосредственно с атомом фосфора и не способен замещатьс€ на катионы.
, следовательно, ћэ(H3PO3) = m(H3PO3)*ћэ( ќЌ)/m( ќЌ)=0.943*56/1.291=41 г/моль. ќсновность H3PO3 равна ћ(H3PO3)/ћэ(H3PO3)=82/41=2, следовательно, образовалась двузамещенна€ соль 2Ќ–O3, данна€ кислота двухосновна€, так как один атом водорода св€зан непосредственно с атомом фосфора и не способен замещатьс€ на катионы.
2 ќЌ + H3PO3 = 2Ќ–O3 + 2Ќ2ќ






