272. ¬ насто€щее врем€ в качестве критери€ самопроизвольного протекани€ реакции используетс€ энерги€ √иббса. ќна определ€етс€ по уравнению: ΔG = ΔH - T*ΔS. ¬ прошлом столетии считалось, что реакци€ протекает самопроизвольно, если она экзотермическа€, т.е. протекает с выделением тепла.
273. Ќа опыте было установлено, что в химических процессах имеют место две тенденции: стремление частиц объединитьс€ за счет более прочных св€зей (уменьшение энтальпии) и стремление частиц перейти в состо€ние большей разупор€доченности (увеличение энтропии). —уммарный эффект этих двух противоположных тенденций отражает изменение энергии √иббса: ΔG = ΔH - T*ΔS. Ёнерги€ √иббса €вл€етс€ критерием самопроизвольного протекани€ реакций, поскольку ее уменьшение €вл€етс€ движущей силой химического процесса: ΔG ≤ 0.
»зменение энтальпии или энтропии не может €вл€тьс€ критерием протекани€ реакции, т.к. часто самопроизвольно протекают реакции, идущие с увеличением энтальпии или уменьшением энтропии.
274. ѕод энтальпийным фактором процесса понимаетс€ его тепловой эффект, т.е. поглощение или выделение тепла при протекании процесса. Ёнтропийный фактор характеризует стремление системы к разупор€дочиванию. ¬ целом самопроизвольное протекание процесса означает увеличение энтропии и/или уменьшение энтальпии. ќба фактора учитывает т.н. энерги€ √иббса: ΔG = ΔH - T*ΔS.
275. Ёнерги€ √иббса: ΔG = ΔH - T*ΔS. Ќесмотр€ на то, что в данное уравнение давление и концентраци€ реагирующих веществ не вход€т,эти характеристики определ€ют значени€ энтальпийного: ΔH вклада и энтропийного вклада T*ΔS, т.е. косвенно вли€ют на энергию √иббса.
276. Ёнерги€ √иббса: ΔG = ΔH - T*ΔS. Ёнерги€ √иббса может дать только приближенный ответ о возможности протекани€ процесса по нескольким причинам: во-первых, энтропи€ и энтальпи€ не посто€нны во всем температурном интервале и во-вторых дл€ протекани€ процесса кроме термодинамической возможности должна выполн€тьс€ и кинетическа€ возможность. инетические параметры процесса уравнение √иббса не учитывает, поэтому и дает приближенный ответ.
277. Ёнерги€ √иббса: ΔG =R*T*lnKp. »з уравнени€ ћенделеева- лайперона ( 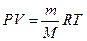 ) можно выразить произведение R*T как функцию давлени€: ΔG =P*V*n*lnKp - при изменении давлени€ газов и концентрации веществ.
) можно выразить произведение R*T как функцию давлени€: ΔG =P*V*n*lnKp - при изменении давлени€ газов и концентрации веществ.
278.
| «нак изменени€ функции | ¬ывод о возможности самопроизвольности протекани€ процесса | |||
| ΔH | ΔS | ΔG | ||
| + | + | + | –еакци€ не протекает | N2O4 (г) = 2 NO2 (г) |
| - | + | - | ѕротекает | 2H2O2(р) = 2H2O (ж) + O2 (г) |
| + | - | + | Ќе протекает | N2(г)+O2(г) = 2NO2(г) |
| - | - | - | ѕротекает | 3H2(г)+N2(г) = 2NH3(г) |
“ = 298 .
279. Ёнерги€ √иббса: ΔG = ΔH - T*ΔS. Ёнерги€ √иббса €вл€етс€ критерием самопроизвольного протекани€ реакции. ≈сли ее изменение отрицательно (ΔG<0), процесс протекает самопроизвольно, если ΔG>0, то процесс термодинамически невозможен. јнализ уравнени€ показывает, что при низких температурах на знак ΔG в большей степени вли€ет ΔЌ, а при высоких температурах - ΔS. Ќапример: 3H2(г)+N2(г) = 2NH3(г), при “=298 ΔЌ<0, ΔS<0, T*ΔS<0 и ΔG<0. ѕри “=498 энтропийный фактор (T*ΔS) возрастает и ΔG>0.
|
|
|
280. а) CaCO3(к) = CaO(к) + CO2(г), энтропи€ возрастает, т.к. в процессе реакции возрастает количество газообразных веществ. ΔSо = ΔSо(—O2) + ΔSо(CaO) - ΔSо(CaCO3) = 213.7 + 39.7 Ц 92/9 = 160.5 ƒж/(K*моль)
б) NH3(г) + HCl(г) = NH4Cl(г), энтропи€ уменьшаетс€, т.к. в процессе реакции уменьшаетс€ количество газообразных веществ. ΔSо = ΔSо(NH4Cl) Ц [ΔSо(HCl) + ΔSо(NH3)] = 95.8 Ц 186.8 Ц 192.6 = -283.6 ƒж/(K*моль)
281. 2SO2(г) + O2(г) = 2SO3(г). ¬ неизолированной системе: ΔG < 0. ΔG = 2ΔGо(SO3) - 2ΔGо(SO2) = 2* (-370) Ц 2*(-300.2) = -139.6 кƒж/моль. ¬ изолированной системе: ΔSо > 0. ΔSо = 2ΔSо(SO3) Ц [2ΔSо(SO2) - ΔSо(ќ2)] = 2*256.4 Ц 205 Ц 2*248.1 = -188.4 ƒж/(K*моль). –еакци€ в пр€мом направлении самопроизвольно протекает в неизолированной системе.
282. —амопроизвольное протекание реакции определ€етс€ в неизолированной системе знаком ΔG, а в изолированной системе знаком ΔS.
| ј) ZnS(к) + 3/2O2(г) = ZnO(к) + SO2(г) ΔGо = ΔGо(SO2) + ΔGо(ZnO) - ΔGо(ZnS) = -300.2 - 320.7 + 200.7 = -420.7 кƒж/моль ΔSо = ΔSо(SO2) + ΔSо(ZnO) - ΔSо(ZnS) = 248.1 + 43.6 - 3/2*205.0 - 57.7 = -73.5 ƒж/(K*моль) –еакци€ протекает самопроизвольно в неизолированной системе, а в изолированной не протекает. |
| Ѕ) AgNO3(к) = Ag(к) + NO2(г) + 1/2O2(г) ΔGо = ΔGо(NO2) - ΔGо(AgNO3) = 51.5 + 33.6 = 85.1 кƒж/моль ΔSо = ΔSо(Ag) + ΔSо(NO2) + 1/2ΔSо(O2) - ΔSо(AgNO3) = 42.6+240.2+1/2*205-14-.9 = 244.4 ƒж/(Kмоль) –еакци€ протекает самопроизвольно в изолированной системе, а в неизолированной не протекает. |
| ¬) CuCl2(к) + H2O(г) = CuO(к) + 2HCl(г) ΔGо = 2ΔGо(HCl)+ΔGо(CuO)-ΔGо(CuCl2)-ΔGо(H2O) = -2*94.8-129.4+228.6+174.1 = 83.7 кƒж/моль ΔSо = 2ΔSо(HCl)+ΔSо(CuO)-ΔSо(CuCl2)-ΔSо(H2O) = 2*186.8+42.6-188.7-108.1 = 119.4 ƒж/(Kмоль) –еакци€ протекает самопроизвольно в изолированной системе, а в неизолированной не протекает. |
283. —амопроизвольное протекание реакции определ€етс€ в неизолированной системе знаком ΔG, а в изолированной системе знаком ΔS.
| ј) SO2(г) + 2H2S(г) = 3S(ромб) + 2H2O(ж) ΔGо = 2ΔGо(H2O) - 2ΔGо(H2S) - ΔGо(SO2) = -2*237.1 + 2*33.8 + 300.2 = -106.4 кƒж/моль ΔSо = 2ΔSо(H2O)+3ΔSо(S)-2ΔSо(H2S)-ΔSо(SO2) = 2*70.1+3*31.9-2*205.7+248.1 = -422.2 ƒж/(K*моль) –еакци€ протекает самопроизвольно в неизолированной системе, а в изолированной не протекает. |
| Ѕ) PbS(г) + 3/2O2(г) = PbO(к) + SO2(г) ΔGо = ΔGо(SO2) + ΔGо(PbO) - ΔGо(PbS) = -300.2 - 189.1 + 98.8 = -390.5 кƒж/моль ΔSо = ΔSо(SO2)+ΔSо(PbO)-ΔSо(PbS)-3/2ΔSо(O2) = 248.1+66.2-91.2-3/2*205 = -84.5 ƒж/(K*моль) –еакци€ протекает самопроизвольно в неизолированной системе, а в изолированной не протекает. |
| ¬) 3NiO(к) + 2Al(к) = 3Ni(к) + Al2O3(к) ΔGо = ΔGо(Al≠2O3) - 3ΔGо(NiO) = -1582 + 211 = -1371 кƒж/моль ΔSо = 3ΔSо(Ni)+ΔSо(Al2O3)-3ΔSо(NiO)-2ΔSо(Al) = 3*29.9+50.9-2*28.4-3*38 =-30.2 ƒж/(K*моль) –еакци€ протекает самопроизвольно в неизолированной системе, а в изолированной не протекает. |
284. NiO(к) + Pb(к) = Ni(к) + PbO(к), T=800 K, ΔG = ΔH - T*ΔS.
|
|
|
ΔHо = ΔHо(PbO) - ΔHо(NiO) = -219.3 Ц(-239.7) = 20.4 кƒж/моль.
ΔSо = ΔSо(PbO)+ΔSо(Ni)-ΔSо(NiO)-ΔSо (Pb) = 66.2 + 29.9 Ц 38 Ц 64.8 = -6.7 ƒж/(K*моль)
ΔG = 20400 Ц 800*(-6.7)= 25.76 кƒж/моль. ΔG>0, поэтому реакци€ идет самопроизвольно в обратном направлении.
285. Cu(к) + ZnO(к) = Zn(к) + CuO(к), T=400 K, ΔG = ΔH - T*ΔS.
ΔHо = ΔHо(CuO) - ΔHо(ZnO) = -162 Ц(-350.3) = 188.6 кƒж/моль.
ΔSо = ΔSо(CuO)+ΔSо(Zn)-ΔSо(ZnO)-ΔSо (Cu) = 42.6 + 41.6 Ц 43.6 Ц 33.2 = 7.4 ƒж/(K*моль)
ΔG = 188600 Ц 400*7.4= 185.64 кƒж/моль. ΔG>0, поэтому реакци€ в пр€мом направлении не протекает.
286. KOH + Na = NaOH + K, T=723 K, ΔG = ΔH - T*ΔS.
ΔHо = ΔHо(NaOH) - ΔHо(KOH) = -426 + 425.8 = -0.2 кƒж/моль.
ΔSо = ΔSо(NaOH)+ΔSо(K)-ΔSо(KOH)-ΔSо (Na) = 64.4 + 71.5 Ц 51.2 Ц 79.3 = 5 ƒж/(K*моль)
ΔG = -200 Ц 723*5= -3.82 кƒж/моль. ΔG<0, поэтому реакци€ возможна при данных услови€х.
287. KCl + Na = NaCl + K, T=1073 K, ΔG = ΔH - T*ΔS.
ΔHо = ΔHо(NaCl) - ΔHо(KCl) = -411.1 - (-435.9) = 24.8 кƒж/моль.
ΔSо = ΔSо(NaCl)+ΔSо(K)-ΔSо(KCl) - ΔSо (Na) = 72.1 + 71.5 Ц 51.2 Ц 82.6 = 9.8 ƒж/(K*моль)
ΔG = 24800 Ц 723*9.8= 14.28 кƒж/моль. ΔG<0, поэтому реакци€ не возможна при данных услови€х. –еакци€ возможна при “= 2530 , чот подтверждаетс€ рассчетом: ΔG = 0, “ = ΔH / ΔS. “ = 24800 / 9.8 = 2530 = 2257 0—.
288. N2 + O2 = 2NO. –еакци€ возможна при условии ΔG<=0, поэтому ΔH = T*ΔS. “=ΔHо/ΔSо. ΔHо = 2ΔHо(NO) = 2*90.2 = 180.4 кƒж/моль. ΔSо = 2ΔSо(NO) - ΔSо(N2) - ΔSо(O2) = 2*210.6 - 191.5 - 205.0 = 24.7 ƒж/(K*моль). T = 180400/24.7 = 7304K = 7031 oC. ƒл€ осуществлени€ этой реакции требуетс€ очень высока€ температура, поэтому она в промышленности не реализована.
289. а)FeO + C(графит) = Fe + CO, T1= 273 K, T2= 1273 K. ΔG = ΔH - T*ΔS.
ΔHо = ΔHо(—ќ) - ΔHо(FeO) = -110.5 + 264.8 = 154.3 кƒж/моль
ΔSо = ΔSо(CO)+ΔSо(Fe)-ΔSо(FeO)-ΔSо(C) = 197.5+27.3-60.8-5.7 = 158.3 ƒж/(Kмоль)
ΔGо1 = 154.3*103 - 273*158.3 = 107.13 кƒж/моль
ΔGо2 = 154.3*103 - 1273*158.3 = - 47.22 кƒж/моль
–еакци€ реализуетс€ при 1273 .
б) FeO + Cќ = Fe + CO2, T1= 273 K, T2= 1273 K. ΔG = ΔH - T*ΔS.
ΔHо = ΔHо(—ќ2) Ц [ΔHо(FeO)+ ΔHо(—ќ)] = -393.3 +110.5 + 264.8 = - 18.2 кƒж/моль
ΔSо = [ΔSо(CO2)+ΔSо(Fe)] Ц [ΔSо(FeO)-ΔSо(CO)] = 27.3 + 213.7 - 197.5-60.8 = -17.3 ƒж/(Kмоль)
ΔGо1 = -18.2*103 - 273*(-17.3) = -13.04 кƒж/моль
ΔGо2 = -18.2*103 - 1273*(-17.3) = 3.82 кƒж/моль
–еакци€ реализуетс€ при 273 .
290. а) Cu + 0.5O2 = CuO(к), T = 573 K, ΔG = ΔH - T*ΔS.
ΔHо = ΔHо(—uO) = -162 кƒж/моль
ΔSо = ΔSо(CuO)-[ΔSо(Cu)+0.5Sо(O2)] = 42.6 Ц33.2 Ц 0.5*205 = - 93.1 ƒж/(Kмоль)
ΔGо = -162*103 - 573*(-93.1) = -108.65 кƒж/моль
б) 2Cu + 0.5O2 = Cu2O(к), T = 573 K
ΔHо = ΔHо(—u2O) = -173.2ƒж/моль
ΔSо = ΔSо(Cu2O)-[2ΔSо(Cu)+0.5Sо(O2)] = 92.9 Ц2*33.2 Ц 0.5*205 = -76 ƒж/(Kмоль)
ΔGо = -173.2*103 - 573*(-76) = -129.65 кƒж/моль
Ѕолее веро€тна в данных услови€х реакци€ б), т.к. ΔGоб) < ΔGоа) .
291. а) NH4NO3(к) = N2O(г) + 2H2O(г), “ = 500 . ΔG = ΔH - T*ΔS.
ΔHо = 2ΔHо(H2O) + ΔHо(N2O) - ΔHо (NH4NO3)= 2*(-241.8)+82 + 365.4 = -36.2 кƒж/моль.
ΔSо = 2ΔSо(H2O)+ΔSо(N2O)-ΔSо(NH4NO3) = 2*188.7 +219.9 - 151 = 446.3 ƒж/(K*моль)
ΔG = -36.2*103 Ц 500*446.3 = -259.35 кƒж/моль.
|
|
|
б) NH4NO3(к) = N2(г) + 0.5ќ2 + 2H2O(г),
ΔHо = 2ΔHо(H2O) - ΔHо (NH4NO3)= 2*(-241.8) + 365.4 = -118.2 кƒж/моль.
ΔSо=2ΔSо(H2O)+ΔSо(N2)+0.5ΔSо(ќ2)-ΔSо(NH4NO3)=2*188.7+191.5+0.5*205-151=520.4 ƒж/(K*моль)
ΔG = -118.2*103 Ц 500*520.4 = -378.4 кƒж/моль.
Ѕолее веро€тна в данных услови€х реакци€ б), т.к. ΔGоб) < ΔGоа) .
292. “= 300 , ΔG = ΔH - T*ΔS.
а) CaCl2(к) + F2(г) = CaF2(к) + Cl2(г)
ΔHо = ΔHо(CaF2) - ΔHо(CaCl2) = -1214.6+795= - 419.6 ƒж/моль.
ΔSо = ΔSо(CaF2)+ΔSо(Cl2)-[ΔSо(CaCl2)+ ΔSо(F2)] = 222.9+68.9Ц202.9-113.6= - 24.7 ƒж/(K*моль)
ΔG = -419.6*103 Ц 300*(-24.7) = - 412.19 кƒж/моль.
б) CaF2 (к) + Cl2 (г) = CaCl2 (к) + F2 (г)
ΔHо = ΔHо(CaCl2)- ΔHо(CaF2)= -795+1214.6= 419.6 ƒж/моль.
ΔSо = ΔSо(CaCl2)+ ΔSо(F2)-[ΔSо(CaF2)+ΔSо(Cl2)] = 202.9+113.6-222.9-68.9= 24.7 ƒж/(K*моль)
ΔG = 419.6*103 Ц 300*24.7 = 412.19 кƒж/моль.
¬озможна реакци€ а), т.к. ΔGоа) < 0, невозможна реакци€ б), т.к. ΔGоб) > 0.
293. T=300 K, ΔG = ΔH - T*ΔS.
| ј) H2S(г) + Cl2(г) = S(ромб) + 2HCl(г) ΔHо = 2ΔHо(H—l) - ΔHо(H2S) = -2*91.8 + 21 = 162.6 кƒж/моль ΔSо = 2ΔSо(HCl)+ΔSо(S)-ΔSо(H2S)-ΔSо(Cl2) = 2*186.8+31.9-205.7-222.9 = -23.1 ƒж/(Kмоль) ΔGо = 162.6*103 + 300*23.1 = 169.53 кƒж/моль |
| Ѕ) H2S(г) + Y2(г) = S(ромб) + 2HY(г) ΔHо = 2ΔHо(HY) - ΔHо(H2S) = 2*26.6 + 21 = 74.2 кƒж/моль ΔSо = 2ΔSо(HY)+ΔSо(S)-ΔSо(H2S)-ΔSо (Y2) = 2*206.5 + 31.9 - 260.6 - 205.7 = -21.4 ƒж/(Kмоль) ΔGо = 74.2*103 + 300*21.4 = 80.62 кƒж/моль |
Ѕолее веро€тна реакци€ б), т.к. ΔGо(б)<ΔGо(а).
294. Ёнерги€ √иббса Ц термодинамическа€ функци€ состо€ни€ системы, котора€ характеризует направление и предел самопроизвольного протекани€ реакции, протекающей при –=const и температуре “. —тандартной энергией √иббса образовани€ вещества называют энергию √иббса реакции образовани€ 1 моль этого вещества, наход€щегос€ в стандартном состо€нии, из соответствующих простых веществ, также наход€щихс€ в стандартных и термодинамически устойчивых при данной “-ре фазах и модификаци€х. Ќапример:
Na2O(тв.) + H2O(ж) = 2NaOH(тв.)
ΔGо = 2ΔGо(NaOH) - ΔGо(H2O) - ΔGо(Na2O) = 2*(-380,45) Ц (-237,4) Ц (-377,38) = -146,12 кƒж/моль
–еакци€ протекает в пр€мом направлении, т.к. ΔGо< 0.
295. а) 2N2O + O2 = 4NO, ΔGо = 4ΔGо(NO) - 2Gо(N2O) = 4*86.6 Ц 2*104/2 = 138 кƒж/моль
б)N2O + NO=NO2 + N2, ΔGо=ΔGо(NO2)Ц[Gо(N2O)+ΔGо(NO)]=51.5-86.6-104.2 =-139.3 кƒж/моль
в)N2O + NO2 =3 NO, ΔGо=3ΔGо(NO)Ц[Gо(N2O)+ΔGо(NO2)]=3*86.6-51.5-104.6=104.1 кƒж/моль
г) CH4 + 3CO2 = 4CO + 2H2O(г), ΔGо=2ΔGо(H2O)+ 4ΔGо(—O)Ц[3Gо(—ќ2)+ΔGо(CH4)]= 2*(-228.6)+4*(-137.1)-3*(-394.4)-(-50.8)= 228.4 кƒж/моль
—амопроизвольно в стандартных услови€х протекает реакци€ б), т.к. ΔGоб) < 0
296.
а) 4HCl + O2 = 2Cl2 + H2O ΔGо = 2ΔGо(H2O) - 4ΔGо(HCl) = -2*228.6 + 4*94.8 = -78 кƒж/моль
б) 2HF + O3 = F2 + O2 + 2H2O ΔGо = -2*228.6 + 2*272.8 = 88.4 кƒж/моль
в) H2O2 + O3 = 2O2 + H2O ΔGо = ΔGо(H2O) - ΔGо(H2O2) = -237.2 + 120.4 = -116.8 кƒж/моль
г) — + H2O = CO + H2 ΔGо = ΔGо(CO) - ΔGо(H2O) = -137.1 + 228.6 = 91.5 кƒж/моль
¬ стандартных услови€х самопроизвольно протекают реакции а) и в), т.к. ΔGо < 0
297.
| N2 + O2 = 2NO |
| ΔHо = 2ΔHо(NO) = 2*90.2 = 180.4 кƒж/моль ΔSо = 2ΔSо(NO) - ΔSо(N2) - ΔSо(O2) = 2*210.6 - 191.5 - 205.0 = 24.7 ƒж/(Kмоль) |
| T1 =1273 K ΔGо = ΔHо - TΔSо = 18040 - 1273*24.7 = 179.16 кƒж/моль T2 =2273 K ΔGо = ΔHо - TΔSо = 18040 - 2273*24.7 = 124.26 кƒж/моль T3 =3273 K ΔGо = ΔHо - TΔSо = 18040 - 3273*24.7 = 99.56 кƒж/моль T4 =5273 K ΔGо = ΔHо - TΔSо = 18040 - 5273*24.7 = 50.16 кƒж/моль T5 =10273 K ΔGо = ΔHо - TΔSо = 18040 - 10273*24.7 = -73.34 кƒж/моль |
 ƒл€ самопроизвольного протекани€ реакции требуетс€ около 7400
ƒл€ самопроизвольного протекани€ реакции требуетс€ около 7400
|
298. “ермодинамически устойчивые вещества характеризуютс€ отрицательным значением энергии √иббса (ΔG), а термодинамически неустойчивые соединени€ - положительным значением ΔG. —оединени€ второго типа существуют, т. к. дл€ их разрушени€ существуют кинетические затруднени€ - скорость разложени€ очень мала. Ќеустойчивые термодинамически вещества получают не пр€мым синтезом, а косвенным путем. “ак, например, получают неустойчивые вещества —2Ќ2, N2O3, Cl2O7 и другие.
|
|
|
299. 2Ќ2ќ2 = 2Ќ2ќ + ќ2. ѕероксид водорода разлагаетс€ при комнатной температуре, т.к. при этом получаютс€ еще более устойчивые вещества. ΔGреакции = 2ΔG(Ќ2ќ) - 2ΔG(Ќ2ќ2) = -2*241.8 + 2*187.8 = -108 кƒж/моль, т.е. реакци€ термодинамически возможна.
300. Ёнергию √иббса часто называют Ђсвободнойї энергией, т.к. она €вл€етс€ функцией давлени€ и температуры, т.е. параметров, которые можно свободно мен€ть и фиксировать. ƒл€ окислительно-восстановительных реакций энерги€ √иббса определ€етс€ уравнением: ΔG = -z*F*E, где z - количество электронов, F - посто€нна€ ‘араде€, E - электродвижуща€ сила.
’имическа€ кинетика.
301. —корость химической реакции по некоторому компоненту называетс€ изменение количества этого компонента в единицу времени в единице реакционного пространства. Ќа практике скорость реакции может определ€тьс€ по различным параметрам: по изменению концентрации одного из веществ, по изменению цветности, рЌ, электропроводности и др. —корость реакции - это кинетическа€ характеристика, а энерги€ √иббса - термодинамическа€ характеристика, эти параметры между собой не св€заны. “.к. скорость реакции определ€етс€ концентраци€ми реагирующих веществ, которые посто€нно измен€ютс€, то ее определ€ют на данный момент времени.
302. ќсновным пон€тием химической кинетики €вл€етс€ пон€тие о скорости химической реакции. ћатематическое выражение скорости реакции зависит от типа реакции: гетерогенна€ или гомогенна€. ѕоэтому в кинетике проводитс€ такое разделение реакций.
гомогенные: N2(г)+O2(г) = 2NO2(г), N2O4 (г) = 2 NO2 (г);
гетерогенные: Ca(тв) + 2H2O(ж) = Ca(OH)2(ж) + H2(г), CuO(тв) = Cu(тв) + 1/2O2(г).
303. ќдной из основных задач химической кинетики €вл€етс€ нахождение зависимости скорости реакции от концентраций реагирующих веществ (кинетическое уравнение). ƒл€ простой реакции кинетическое уравнение находитс€ легко, в то врем€ как дл€ сложной реакции нахождение кинетического уравнени€ представл€ет собой очень сложную задачу. ѕоэтому прин€то разделение реакций на простые и сложные.
ѕростые реакции. ћоно: Cl2 = 2Cl, Ѕи: Ќ+ + —l- = HCl, “ри: 3O2 = 2O3.
—ложные реакции: ѕоследовательные: 2Ca + O2 = 2CaO => CaO + H2O = Ca(OH)2, ѕараллельные: Ca + Cl2 = CaCl2 и Ca + F2 = CaF2, ÷епные: —l2 = 2Cl*, Cl* + CH4 = CH3* + HCl, CH3* + Cl2 = CH3Cl + Cl* и т.д.
ѕо стехиометрическому уравнению нельз€ однозначно определить - сложна€ это реакци€ или проста€, но если в реакцию вступают более трех частиц, то эта реакци€ сложна€.
304. Ќа скорость реакции вли€ют: природа взаимодействующих частиц, температура, давление, катализатор и др.
305. инетическое уравнение дл€ простой и сложной реакции записываетс€ как зависимость скорости реакции от концентрации взаимодействующих веществ. ќднако, если дл€ простой реакции кинетическое уравнение имеет относительно простой вид, то дл€ сложных реакций кинетическое уравнение может записыватьс€ в виде системы уравнений. онстанта скорости реакции - это скорость реакции при концентрации веществ равных единице. Ќа константу скорости вли€ет температура, давление, природа веществ и не вли€ет концентраци€ веществ и объем системы.
306. а) 2HI = H2 + I2, V = *–2(HI) , после увеличени€ давлени€ в 2 раза V/ = *(2–(HI))2 , в результате скорость возрастет в V/ / V = 4 раза.
Ѕ) N2O4 = 2NO2, V = *– (N2O4), после увеличени€ давлени€ в 2 раза V/ = *(2–(N2O4)), в результате скорость возрастет в V/ / V = 2 раза.
¬) 2NO +H2 = N2O + H2O, V = *–2(N2O) *P(H2), после увеличени€ давлени€ в 2 раза V/ = *(2–(N2O)) 2 *2P(H2), в результате скорость возрастет в V/ / V = 8 раз.
307. a) 2NO +Cl2 = 2NOCl, V = *–2(NO)*P(Cl2), после увеличени€ давлени€ в 3 раза V/ = *(3–(NO))2 * 3P(Cl2), в результате скорость возрастет в V/ / V = 27 раз.
|
|
|
Ѕ) H2 + OH= H2O + H, V = *–(OH)*P(H2), после увеличени€ давлени€ в 3 раза V/ = *3–(OH)*3P(H2), в результате скорость возрастет в V/ / V = 9 раз.
¬) Cl2 = 2Cl, V = *P(Cl2), после увеличени€ давлени€ в 3 раза V/ = *3P(Cl2), в результате скорость возрастет в V/ / V = 3 раза.
308. а) O2+H = OH + O, V = *–(H) *P(O2), после увеличени€ давлени€ в 4 раза V/ = *4–(H) *4P(O2), в результате скорость возрастет в V/ / V = 16 раз.
Ѕ) H2+Y2 = 2HY, V = *–(H2)*P(Y2), после увеличени€ давлени€ в 4 раза V/ = *4–(H2)*4P(Y2), в результате скорость возрастет в V/ / V = 16 раз.
¬) 2NO+O2 = 2NO2, V = *–2(NO2)*P(O2), после увеличени€ давлени€ в 4 раза V/ = *(4–(NO2) )2 *4P(O2), в результате скорость возрастет в V/ / V = 64 раза.
309. H2+Br2 = 2HBr, V = K*[H2]*[Br2]1/2 .ћолекул€рность реакции равна1+1 =2, а пор€док реакции 1+1/2=1,5. ѕоскольку молекул€рность и пор€док не совпадают, то данна€ реакци€ - сложна€. ј) V/=K*4[H2]*[Br2]1/2 , скорость реакции возрастает в V/ / V= 4 раза.
Ѕ) V//=K*[H2]*(4[Br2])1/2 , скорость реакции возрастает в V// / V= 2 раза.
¬) V///=K*4[H2]*(4[Br2])1/2 , скорость реакции возрастает в V// / V= 8 раз.
310. F2+ 2ClO2=2ClO2F, V=K*[F2]*[ClO2], ƒанна€ реакци€ €вл€етс€ сложной, т.к. ее пор€док (2) и молекул€рность (3) не совпадают. ѕри увеличении давлени€ в 3 раза: V/ = K*3[F2]*3[ClO2], скорость реакции возрастает в V/ / V = 9 раз.
311. 2NO +2H2 = N2 + 2H2O, а) фиксируем концентрацию [H2] = 0,12 моль/л. ѕри увеличении [NO] в 2 раза, скорость реакции возрастает в 4 раза, т.е. пор€док по[NO] равен 2. б) фиксируем [NO] = 1,12 моль/л. ѕри увеличении [H2] в 2 раза, скорость возрастает в 2 раза - пор€док по [H2] = 1. “.о. кинетическое уравнение имеет вид: V = K*[H2]1 *[NO]2.
312. SO2+2H2=S+2H2O, а) фиксируем концентрацию [SO2]=200. ѕри увеличении [H2] в 2 раза, скорость реакции возрастает в 2 раза, т.е. пор€док по[H2] равен 1. б) фиксируем [H2]=200. ѕри увеличении [SO2] в 2 раза, скорость возрастает в 2 раза - пор€док по [SO2]=1. “.о. кинетическое уравнение имеет вид: V=K*[H2]1 *[SO2]1.






