Стехиометрические расчеты
211. а) FeO - возьмем 1 моль оксида, тогда его масса m(FeO) = n*M = 1*72 = 72 г. Содержание железа и кислорода в оксиде n(Fe) = n(O) = 1 моль.
m(Fe) = n*M = 1*56 = 56 г. w%(Fe) = m(Fe)*100%/m(FeO) = 56*100%/72 = 77.78 %
m(O) = n*M = 1*16 = 16 г. w%(O) = m(O)*100%/m(FeO) = 16*100%/72 = 22.22 %
б) Fe2O3- возьмем 1 моль оксида, тогда его масса m(Fe2O3) = n*M = 1*160 = 160 г. Содержание железа и кислорода в оксиде n(Fe) = 2 моль, n(O) = 3 моль.
m(Fe) = n*M = 2*56 = 112 г. w%(Fe) = m(Fe)*100%/m(Fe2O3) = 112*100%/160 = 70%
m(O) = n*M = 3*16 = 48 г. w%(O) = m(O)*100%/m(Fe2O3) = 48*100%/160 = 30%
212. KMnO4 - возьмем 1 моль соли, тогда её масса m(KMnO4) = n*M = 1*158 = 158 г. Содержание калия, марганца и кислорода: n(K) = 1 моль, n(Mn) = 1 моль, n(O) = 4 моль.
m(K) = n*M = 1*39=39 г. w%(K) = m(K)*100%/m(KMnO4) = 39*100%/158 = 24.7%
m(Mn) = n*M = 1*55 = 55 г. w%(Mn) = m(Mn)*100%/m(KMnO4) = 55*100%/158 = 34.8%
m(O) = n*M = 4*16 = 64 г. w%(O) = m(O)*100%/m(KMnO4) = 64*100%/158 = 40.5%
213. а) NH3 - возьмем 1 моль аммиака, тогда его масса m(NH3)=n*M=1*17=17 г. Содержание азота и водорода: n(N)=1 моль, n(H)=3 моль.
m(N)=n*M=1*14=14 г. w%(N)=m(N)*100%/m(NH3) =14*100%/17=82.4%
m(H)=n*M=3*1=3 г. w%(H)=m(H)*100%/m(NH3) =3*100%/17=17.6%
а) N2H4 - возьмем 1 моль гидразина, тогда его масса m(N2H4)=n*M=1*30=30 г. Содержание азота и водорода: n(N)=2 моль, n(H)=4 моль.
m(N)=n*M=1*14=14 г. w%(N)=m(N)*100%/m(NH3) =14*100%/17=82.4%
m(H)=n*M=3*1=3 г. w%(H)=m(H)*100%/m(NH3) =3*100%/17=17.6%
214. Сульфат натрия Na2SO4. Возьмем один моль соли. В одном моль соли содержится 2 моль натрия, один моль серы и 4 моль кислорода.
m(Na2SO4)=n*M=1*142=142 г.
m(Na)=n*M=2*23=46 г. w%(Na) = m(Na)*100%/m(Na2SO4) = 46*100/142 = 32.4 %
m(S)=n*M=1*32=32 г. w%(S) = m(S)*100%/m(Na2SO4) = 32*100/142 = 22.5 %
m(O)=n*M=4*16=64 г. w%(O) = m(O)*100%/m(Na2SO4) = 64*100/142 = 45.1 %
215. Обезвоженные хромокалиевые квасцы: KCr(SO4)2 Возьмем один моль соли. В одном моль соли содержится 1 моль калия, 1 хрома, 2 моль серы и 8 моль кислорода.
m(KCr(SO4)2)=n*M=1*283=283 г.
m(K)=n*M=1*39=39 г. w%(K) = m(K)*100%/m(KCr(SO4)) = 39*100/283 = 13.8 %
m(Cr)=n*M=1*52=52 г. w%(Cr) = m(Cr)*100%/m(KCr(SO4)) = 52*100/283 = 18.4 %
m(S)=n*M=2*32=64 г. w%(S) = m(S)*100%/m(KCr(SO4)) = 64*100/283 = 22.6 %
m(O)=n*M=8*16=128 г. w%(O) = m(O)*100%/m(KCr(SO4)) = 128*100/283 = 45.2 %
216. w%(H) = 5.882 %, w%(O) = 100-5.882 = 94.118 %. Dвоздуху = 1.1724. Молярная масса вещества равна: M(HxOy) = Dвоздуху*M(воздуха) = 1.1724*29 = 34 г/моль. Возьмем один моль вещества: его масса равна 34 грамма, следовательно -
m(H) = 34*5.882%/100% = 2 г. n(H) = m/M = 2/1 = 2 моль.
m(О) = 34*94.118%/100% = 32 г. n(О) = m/M = 32/16 = 2 моль. Следовательно, это Н2О2.
217. w%(As) = 75.76 %, w%(O) = 100-75.76 = 24.24 %. Dвоздуху = 13.65. Молярная масса вещества равна: M(AsxOy) = Dвоздуху*M(воздуха) = 13.65*29 = 395.85 г/моль. Возьмем один моль вещества: его масса равна 395.85 грамма, следовательно -
m(As) = 395.85*75.76%/100% = 300 г. n(As) = m/M = 300/75 = 4 моль.
m(О) = 395.85*24.24%/100% = 96 г. n(О) = m/M = 96/16 = 6 моль.
Следовательно, это As4О6.
218. w%(B) = 78.2 %, w%(H) = 100-78.2 = 21.8 %. Так как масса одного литра этого газа равна массе одного литра азота, то молекулярные массы этих газов также равны: M(BxHy) = M(N2) = 28 г/моль. Возьмем один моль BxHy: его масса равна 28 г., следовательно -
m(B) = 28*78.2%/100% = 22 г. n(B) = m/M = 22/11 = 2 моль.
m(H) = 28*21.8%/100% = 6 г. n(H) = m/M = 6/1 = 6 моль.
Следовательно, это B2H6.
219. w%(C) = 80 %, w%(H) = 20 %. DH = 15. Молярная масса вещества равна: M(CxHy) = DH*M(H2) = 15*2 = 30 г/моль. Возьмем один моль вещества: его масса равна 30 грамм, следовательно -
m(C) = 30*80%/100% = 24 г. n(C) = m/M = 24/12 = 2 моль.
m(H) = 30*20%/100% = 6 г. n(H) = m/M = 6/1 = 6 моль.
Следовательно, это C2H6.
220. w%(C) = 92.3 %, w%(H) = 7.7 %.
V = 1л, m = 1.17г, M(CxHy) = m/n = m*Vм/V = 1.17*22.4/1 = 26 г/моль.
Возьмем один моль вещества: его масса равна 26 грамм, следовательно -
m(C) = 26*92.3%/100% = 24 г. n(C) = m/M = 24/12 = 2 моль.
m(H) = 26*7.7%/100% = 2 г. n(H) = m/M = 2/1 = 2 моль.
Следовательно, это C2H2.
б) V = 1л, m = 3.51г, M(CxHy) = m/n = m*Vм/V = 3.51*22.4/1 = 78 г/моль.
Возьмем один моль вещества: его масса равна 26 грамм, следовательно -
m(C) = 78*92.3%/100% = 72 г. n(C) = m/M = 72/12 = 6 моль.
m(H) = 78*7.7%/100% = 6 г. n(H) = m/M = 6/1 = 6 моль.
Следовательно, это C6H6.
221. CaCO3 = CaO + CO2. m(CaO) = 280 кг. n(CaO) = m/M = 280000/56 = 5000 моль. Согласно уравнению реакции, n(CaCO3) = n(CaO) = 5000 моль. m(CaCO3) = n*M=5000*100= 500кг.
222. CaCO3.MgCO3 = CaO + MgO + 2CO2. m(доломита) = 920г. n(доломита) = m/M = 920/184 = 5 моль. Согласно уравнению реакции, n(CO2) = 2n(доломита) = 2*5 = 10 моль. V(CO2) = Vм(CO2)*n(CO2) = 22.4*10 = 224 литра.
223. FeS + 2HCl = FeCl2 + H2S; 2H2S + 3O2 = 2SO2 + 2H2O
V(O2) = 306л., n(O2) = V/Vм = 306/22.4 = 13.66моль. n(H2S) = 2/3n(O2) = 9.11моль. n(FeS) = n(H2S). m(FeS) = n*M = 9.11*88 = 801г.
224. Zn + 2HCl = ZnCl2 + H2 V(H2) = 280мл. n(H2) = V/Vм = 0.28/22.4 = 0.0125моль. Согласно уравнению реакции, n(Zn) = n(ZnCl2) = n(H2). Масса израсходованного цинка - m(Zn) = n*M = 0.0125*65.4 = 0.8175г. Масса образовавшегося хлорида цинка - m(ZnCl2) = n*M = 0.0125*136 = 1.7г.
225. BaCl2 + 2AgNO3 = Ba(NO3)2 + 2AgCl. m(AgCl) = 287г. n(AgCl) = m/M = 287/143.5 = 2моль. Согласно уравнению реакции, n(AgNO3) = n(AgCl), следовательно, m(AgNO3) = n*M = 2*170 = 340г.
226. KOH + HNO3 = KNO3 + H2O. m(KOH) = 10г. - n(KOH) = m/M = 10/56 = 0.18моль, m(HNO3) = 10г. - n(HNO3) = m/M = 10/63 = 0.16моль. В избытке находится гидроксид калия, nизб(KOH) = 0.18 - 0.16 = 0.02моль. mизб(KOH) = nизб*M = 0.02*56 = 1.12г.
227. 2NaOH + H2SO4 = Na2SO4 + 2H2O. m(NaOH) = 9г. n(NaOH) = m/M = 9/40 = 0.225моль. m(H2SO4) = 10г. n(H2SO4)= m/M = 10/98 = 0.102моль. На реакцию с 0.102 моль серной кислоты, согласно уравнению реакции, требуется 2*0.102 = 0.204 моль NaOH. Следовательно в избытке находится щелочь, и среда раствора будет щелочной.
228. HCl + NH3 = NH4Cl. m(HCl) = 7.3; n(HCl) = m/M = 7.3/36.5 = 0.2моль. m(NH3) = 4.0г. n(NH3) = m/M = 4/17 = 0.24г. Согласно уравнению реакции, вещества реагируют в равных количествах, следовательно, в избытке находится аммиак. n(HCl) = n(NH4Cl). m(NH4Cl) = n*M = 0.2*53.5 = 10.7г.
Не прореагировало 0.24-0.2 = 0.04 моль аммиака - m(NH3) = n*M = 0.04*17 = 0.68г.
229. FeCl3 + 3NaOH = Fe(OH)3 + 3NaCl. Согласно уравнению реакции, вещества реагируют в соотношении 1 к 3, следовательно, с 0.20 моль хлорида железа будет взаимодействовать 3*0.2 = 0.6 моль щелочи. Щелочь находится в недостатке. Израсходуется 0.24/3 = 0.08 моль FeCl3 и образуется такое же количество Fe(OH)3. Останется 0.2 - 0.08 = 0.12 моль FeCl3.
230. S + O2 = SO2. m(S) = 8 г. n(S) = m/M = 8/32 = 0.25 моль. V(O2) = 30 литров. n(O2) = V/Vм = 30/22.4 = 1.14 моль. Из уравнения реакции видно, что сера находится в недостатке. После реакции останется кислород в количестве 1.14 - 0.25 = 0.89 моль. V(O2) = n*Vм = 0.89*22.4 = 20 литров.
231. CaCO3 + 2HCl = CaCl2 + CO2 + H2O. V(CO2) = 571.2л. n(CO2) = V/Vм = 571.2/22.4 = 25.5 моль. Согласно уравнению реакции, n(CaCO3) = n(CO2). m(CaCO3) = n*M = 25.5*100 = 2550 г. С учетом примесей масса известняка будет равна 2550*100%/85% = 3000 г.
232. m(Fe2O3) = 2т. Масса чистого оксида составляет m = 2000000*94%/100% = 1880000 г. n(Fe2O3) = m/M = 1880000/160 = 11750 моль. Из одного моль оксида можно получить два моль железа. m(Fe) = 2*n(Fe2O3)*M(Fe) = 2*11750*56 = 1316000 = 1,32 тонны.
233. Объем смеси 1 м3.
V(H2) = 1*50%/100% = 0.5 м3, n(H2) = V/Vм = 0.5*103/22.4 = 22.3 моль.
V(СH4) = 1*35%/100% = 0.35 м3, n(СH4) = V/Vм = 0.35*103/22.4 = 15.6 моль.
V(СО) = 1*8%/100% = 0.08 м3, n(СО) = V/Vм = 0.08*103/22.4 = 3.6 моль.
V(С2H4) = 1*2%/100% = 0.02 м3, n(С2H4) = V/Vм = 0.02*103/22.4 = 0.9 моль.
H2 + O2 = 2H2O На сжигание водорода потребуется n(O2)= 1/2n(H2) = 22.3/2 = 11.15 моль
СH4 + 2O2 = 2H2O+ СO2 На сжигание метана потребуется n(O2)= 2n(СH4) = 15.6*2 = 31.2 моль
2СО + O2 = 2СО2 На сжигание СО потребуется n(O2)= 1/2n(СО) = 3.6/2 = 1.8 моль
С2H4 + 3O2 = 2H2O + 2СО2 На сжигание С2Н4 потребуется n(O2)= 3n(С2H4) = 0.9*3 = 2.7 моль
Всего будет затрачено 11.15 + 31.2 + 1.8 + 2.7 = 46.85 моль кислорода. V(O2) = n*Vм = 46.85*22.4 = 1049.44 литров. V(воздуха) = V(O2)*100%/21% = 4997.3 литров = 5м3.
234. FeS + 2HCl = FeCl2 + H2S. m(FeS) = 500г. n(FeS) = m/M = 500/88 = 5.7 моль. Согласно уравнению реакции из 5.7 моль сульфида железа можно получить 5.7 моль сероводорода. Однако, образовалось n(H2S) = V/Vм = 112/22.4 = 5 моль. Выход реакции В = 5*100%/5.7 = 87.7%.
235. 3О2 = 2О3. В 100 литрах воздуха содержится V(O2) = 100*21%/100% = 21 литр. n(O2) = V/Vи = 21/22.4 = 0.9375 моль. Согласно уравнению реакции, n(O3) = 2/3n(O2) = 2*0.9735/3 = 0.625 моль. V(O3) = 0.625*22.4 = 14 литров. Выход реакции равен В = 1.4*100%/14 = 10%.
236. Zn + 2H+ = Zn2+ + H2. n(H2) = 6.272/22.4 = 0.28 моль. Согласно уравнению реакции, n(Zn) = n(H2). Масса чистого цинка равна m(Zn) = n*M = 0.28 * 65.4 = 18.3 г. Масса примесей в цинке m = 20.4 - 18.3 = 2.1. Следовательно, содержание примесей w%(примесей) = 2.1*100%/20.4 = 10.3%.
237. С + О2 = СО2. n(CO2) = V/Vм = 5.3*103/22.4 = 236.6 моль. Согласно уравнению реакции, n(С) = n(СО2). Масса чистого углерода m(С) = n*M = 236.6*12 = 2,84 кг. Содержание углерода в угле w%(C) = 2.84*100%/3 = 95%.
238. CaCO3*MgCO3 = CaO + MgO + 2CO2. n(СО2) = V/Vм = 21/22.4 = 0.9375 моль. Согласно уравнению реакции, n(доломита) = 1/2n(CO)2 = 0.9375/2 = 0.469 моль. m(доломита) = n*M = 0.469*184 = 86.25 г. Масса примесей в доломите составляет m = 100 - 86.25 = 13.75 г. w%(примесей) = 13.75*100%/100 = 13.75%.
239. 4FeS2 + 11O2 = 2Fe2O3 + 8SO2. Найдем количество образовавшегося сернистого газа: n(SO2) = V/Vм = 3500*103/22.4 = 156250 моль. Согласно уравнению реакции, n(FeS2) = 1/2n(SO2) = 156250/2 = 78125 моль. m(FeS2) = n*M = 78125*120 = 9375 кг = 9.4 тонн. Содержание пирита в руде - w%(FeS2) = 9.4*100%/100 = 9.4%.
240. Обозначим массу алюминия - х, массу магния - у. х + у =12 г. Уравнение Менделеева-Клайперона: 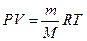 . Количество выделившегося водорода равно: n(H2) = PV/RT = 100.7*14.4/(8.31*(23+273)) = 0.6 моль.
. Количество выделившегося водорода равно: n(H2) = PV/RT = 100.7*14.4/(8.31*(23+273)) = 0.6 моль.
Mg + 2HCl = MgCl2 + H2. n1(H2) = n(Mg) m/M = y/24
2Al + 6HCl = 2AlCl3 + 3H2. n2(H2) = 3/2n(Al) = m/M = x/27
y/24 + x/27 = 0.6 (y = 12 - x), следовательно, (12 - х)/24 + х/27 = 0.6, откуда х=5.9. m(Mg) = 5.9 г. m(Al) = 12 - 5.9 = 6.1 г. Содержание металлов в сплаве равно: w%(Mg) = 5.9*100%/12 = 49.1%, w%(Al) = 100% - 49.2% = 50.2%.
241. Стандартной энтальпией образования (∆Hof) называют тепловой реакции образования одного моля химического соединения из простых веществ, устойчивых при данных условиях. S + O2 = SO2. n(S) = m/M = 10/32 = 0.3125 моль.
∆Hof(SO2) = -Q/n = 92.8/0.3125 = -296.96 кДж/моль. Справочное значение -296.9 кДж/моль
242. Н2 + 1/2О2 = Н2О. n(Н2) = V/Vм = 2.24/22.4 = 0.1 моль. ∆Hof(Н2О) = -Q/n = 28.6/0.1 = -286 кДж/моль. Образовалась вода в жидком состоянии.
243. Ca + F2 = CaF2. n(Са) = m/M = 2/40 = 0.05 моль.
∆Hof(CaF2) = -Q/n = 61/0.05 = 1220 кДж/моль. Ошибка опыта равна (1220-1214.6)/1214.6 = 0.0044 = 0.44%
244. 2Al + 3/2O2 = Al2O3. ∆Hof(Al2О3) = -Q/n, следовательно, n(Al2O3) = 1676/838 = 2 моль. Согласно уравнению реакции, n(Al) = 2n(Al2O3) = 4 моль, n(O2) = 3/2n(Al2O3) = 3 моль. m(Al) = n*M = 4*27 = 108 г. V(O2) = 3*22.4 = 67.2 л.
245. Ca + 1/2O2 = CaO + Q. Q = - ∆Hof * n. ∆Hof(CaO) = -635.5 кДж/моль. n(Ca) = m/M = 200/40 = 5 моль. Q = 635.5*5 = 3177.5 кДж.
246. H2 + Br2 = 2HBr + Q. Q = - ∆Hof * n. ∆Hof(HBr) = -36.3 кДж/моль. n(H2) = V/Vм = 560/22.4 = 25 моль. n(Br2) = m/M = 500/80 = 6.25 моль. Бром находится в недостатке, поэтому расчет ведем по брому: Q = 36.3*6.25 = 227 кДж.
247. Закон Лавуазье-Лапласа: тепловой эффект реакции равен сумме энтальпий образования продуктов реакции за вычетом суммы энтальпий образования исходных веществ. Например: Тепловой эффект реакции CaCO3 = CaO + CO2 запишется как ∆HoР = ∆Ho(CaO) + ∆Ho(CO2) - ∆Ho(CaCO3) = -635.5 - 393.5 + 1206.9 = 177.9 кДж/моль. Закон Лавуазье-Лапласа следует из закона Гесса (тепловой эффект реакции зависит только от вида и состояния исходных веществ и конечных продуктов, но не зависит от пути процесса, т.е. от числа и характера промежуточных стадий), поэтому его называют следствием закона Гесса.
248. CuO = Cu + 1/2O2 - Q. ∆Hof = -Q/n. Q = 12.8 кДж. n(Cu) = m/M =5/64 = 0.078 моль. ∆Hof(CuO) = -12.8/0.078 = -163.8 кДж/моль. Справочное значение: -162 кДж/моль.
249. Cu2O = 2Cu + 1/2O2 - Q. ∆Hof = - Q / n. Q = 17 кДж. n(Cu) = m/M =12.7/64 = 0.2 моль. ∆Hof(Cu2O) = - 2*17 / 0.2 = -171.3 кДж/моль. Справочное значение: -173.2 кДж/моль.
250. CaH2 = Ca + H2 - Q. ∆Hof = - Q / n. Q = 23.6 кДж. V(H2) = V/Vм =2.8/22.4 = 0.125 моль. ∆Hof(CaH2) = - 23.6 / 0.125 = -188.8 кДж/моль. Справочное значение: -188.7 кДж/моль.
251. 1. Са(ОН)2 = СаО + Н2О ∆H01 = 65.3 кДж/моль
2. Ca(OH)2 + SiO2 = CaSiO3 + H2O ∆H02 = -23.3 кДж
3. CaO + SiO2 ∆H03 =?
∆H03 = ∆H02 - ∆H01 = -23.3 – 65.3 = -233.6 кДж.
Закон Гесса: сумма энтальпий двух или более промежуточных реакций при переходе из исходного к конечному состоянию равна энтальпии реакции, которая ведет от исходного к конечному состоянию непосредственно.
1 следствие: теплота образования реакции равна разности между суммой теплот образования продуктов и суммой теплот образования исходных веществ.
2 следствие: теплота сгорания – тепловой эффект реакции сгорания одного моль вещества.
3 следствие: если совершается 2 реакции, приводящие из разных начальных состояний к равным конечным, то разница между их тепловыми эффектами есть тепловой эффект перехода из одного состояния в другое
252. Термохимические уравнения - уравнения, в которых приведены значения энтальпий данной реакции и указаны абсолютные состояния реагентов и продуктов. На основании закона Гесса с термохимическими уравнениями можно оперировать, как и с алгебраическими.
1. H2S + 3/2O2 = H2O + SO2 ∆H01 = -561.1 кДж
2. S + O2 = SO2 ∆H02 = -296.2 кДж
3. 2H2S + SO2 = 3S + 2H2O ∆H03 =?
∆H03 = 2∆H01 - 3∆H02 = 2*(-561.1) - 3(-296.2) = -233.6 кДж.
253. а) 2As(к) + 3F2(г) = 2AsF3(г), ∆H01 = -1842 кДж
б) AsF5(г) = AsF3(г) + F2(г), ∆H02 = 317 кДж
в) 2As(к) + 5F2(г) = 2AsF5(г), ∆H03 =?
∆H03 = ∆H01 - 2∆H02 = -1842 - 2*317 = -2476 кДж.
254.
| 1. Fe2O3 + 3CO = 2Fe + 3CO2 | ∆H01 = -1673.7 кДж |
| 2. C + 1/2O2 = CO | ∆H02 = -110.4 кДж |
| 3. CO2 = C + O2 | ∆H03 = 393.3 кДж |
| 4. 2Fe + 3O2 = Fe2O3 | ∆H04 =? |
| По закону Гесса, ∆H04 = -∆H01 - 3∆H02 - 3∆H03 = 1673.7 + 3*110.4 - 3*393.3 = 825 кДж |
255. а) CH4 + 2O2 = CO2 + 2H2O, ∆H01 = -892 кДж
б) 2CH3Cl + 3O2 = 2CO2 + 2H2O() + 2HCl(), ∆H02 = -1374 кДж
в) 2H2 + O2 = 2H2O, ∆H03 = -571,7 кДж
г) H2+ Cl2 = 2HCl, ∆H04 = -185 кДж
д) CH4 + Cl2 = CH3Cl + HCl, ∆H05 =?
∆H05 = (2∆H01 - ∆H02 - ∆H03 +2∆H04) / 2 = [2*(- 892) – (-1374) – (-571.7) + 2*(-185)] / 2 = -104.15 кДж
256. а) Na2CO3 + SiO2 = Na2SiO3 + CO2, ∆H01 = 819.3 кДж
б) Na2O + SiO2 = Na2SiO3, ∆H02 = -243.5 кДж
в) Na2SiO3 = Na2O + CO2, ∆H03 =?
Q = - n*∆H03, ∆H03 = ∆H01 - ∆H02 = 819.3 – (-243.5) = 1062.8 кДж. n(Na2CO3) = m / M = 200/106 = 1/89 моль. Q = - 1.89 * 1062.8 = - 2008.7 кДж.
257. Основной закон термохимии - закон Гесса: тепловой эффект реакции зависит только от вида и состояния исходных веществ и конечных продуктов, но не зависит от пути процесса, т.е. от числа и характера промежуточных стадий. Следствие: тепловой эффект реакции равен сумме энтальпий образования продуктов реакции за вычетом суммы энтальпий образования исходных веществ. В математическом виде: ∆Hoреакции = ∑ ∆Ho(продуктов)- ∑ ∆Ho (исходных веществ). CH4+2O2 = CO2+2H2O. ∆Hoреакции = ∆Ho(СО2) + 2∆Ho(Н2О) - ∆Ho(СН4) - 2∆Ho(О2) = -393.5-2*241.8+74.8 = -802.3 кДж/моль. n(CH4) =V/Vм = 1000/22.4 = 44.64 моль. Выделится тепла: Q = - ∆Ho*n = 802.3*44.64 = 35817 кДж.
258. ∆Hoреакции = 2∆Ho(MgО) + ∆Ho(C) - 2∆Ho(Mg) - ∆Ho(CО2) = -2*601.8 + 393.5 = -810.1 кДж/моль. n(Mg) = m/M = 1000/24 = 41.67 моль. Выделится тепла: Q = - ∆Ho*n = 810.1*41.67 = 33754.2 кДж.
259. ∆Hoреакции = ∆Ho(KCl) + ∆Ho(O2) - ∆Ho(KClO3) = -435.9 + 391.2 = -44.7 кДж/моль. n(KClO3) = m/M = 1000/122.5 = 8.2 моль. Выделится: Q = - ∆Ho*n = 44.7*8.2 = 365 кДж.
260. ∆Hoреакции = ∆Ho(N2O5) - ∆Ho(O2) – 2∆Ho(NO) = (-42.7) –2*90.2 = -223.1кДж/моль. n(N2O5) = m/M = 1000/108 = 9.3 моль. Выделится: Q = - ∆Ho*n = 223.1*9.3 = 2074.8 кДж.
261. Ca + 2H2O = Ca(OH)2 + H2, ∆Hoреакции = ∆Ho(Ca(OH)2) - 2∆Ho(H2O) = -986.6 + 2*285.3 = - 416 кДж/моль. n(H2) = V/Vм = 1000/22,4 = 44,64моль. Q = - ∆Ho*n(H2) = - 416 * 44,64 = 18570 кДж.
262. ∆Hoреакции = ∆Ho(SO2) + ∆Ho(H2O) - ∆Ho(H2S) = -296,9 - 241,8 + 21 = -517,7 кДж/моль. n(H2S) = V/Vм = 1000/22,4 = 44,64моль. Выделится: Q = - ∆Ho*n = 44.64*-517,7 = -23110,13кДж.
263. C2H4 + 3O2 = 2CO2 + 2H2O, ∆Hoреакции = 2∆Ho(СО2) + 2∆Ho(Н2О) - ∆Ho(С2Н4) = -393.5*2-2*241.8 - 52,3 = -1322,9кДж/моль. n(C2H4) =V/Vм = 1000/22.4 = 44.64 моль. Выделится тепла: Q = - ∆Ho*n = 1322,9*44.64 = 59054,3 кДж.
264. C2H5OH + 3O2 = 2CO2 + 3H2O, ∆Hoреакции = 2∆Ho(СО2) + 3∆Ho(Н2О) - ∆Ho(C2H5OH) = -393.5*2-3*241.8+227,6= -1288,4 кДж/моль. n(C2H4) =V/Vм = 1000/22.4 = 44.64 моль. Выделится тепла: Q = - ∆Ho*n = 1288,4*44.64 = 57514,2 кДж.
265. Ca(OH)2 = H2O + CaO, Hoреакции = ∆Ho(H2O) + ∆Ho (CaO) - ∆Ho(Ca(OH)2) = -285,3-635,5 + 986,6 = 65,6 кДж/моль. n(H2O) = m/M = 1000/18 = 55,56 моль. Теплоты поглотилось: Q = - ∆Ho*n(H2) = - 65,6*55,56 = -3086,6 кДж.
266. CaCO3 = CaO + CO2, Hoреакции = ∆Ho(CO2) + ∆Ho (CaO) - ∆Ho(CaCO3) = -393,5 - 635,5 + 1206,9 = 177,9 кДж/моль. n(CO2) = V/Vм = 100000/22.4 = 4464,28 моль. Теплоты поглотилось: Q = - ∆Ho*n(CO2) = - 177,9*4464,28 = - 794196 кДж.
267. Стандартная энтальпия сгорания характеризует изменение энтальпии, происходящее при сгорании 1 моль вещества. C2H6 + H2 = 2C2H4. ∆Hреакции = 2∆Hсгорания (С2Н4) - (∆Hсгорания(H2) + ∆Hсгорания(С2H6)) = 2*1411,0 - 241,8 - 1599,9 = 980,3 кДж/моль.
268. ∆Hoреакции = ∆Hсгорания(С2Н6) - ∆Hсгорания(С2H4) - ∆Hсгорания(H2) = 1599.9 - 1411.0 - 241,8 = -52.9 кДж/моль.
269. ∆Hoреакции = ∆Hсгорания(С6Н6) - 3 ∆Hсгорания(С2H2) = -3268 + 3*1301 = 635 кДж/моль.
270. Теплотворная способность топлива - это количество теплоты, выделяемое при сгорании определенного количества топлива, например, одного кг или литра. 60% Н2 и 40% СН4. В одном литре содержится 0.6 л. водорода и 0.4 литра метана. n(H2) = V/Vм = 0.6/22.4 = 0.027 моль. n(CH4) = 0.4/22.4 = 0.018 моль. При сгорании одного литра данной смеси выделится теплоты: Q = -n(H2)*∆Ho(H2) - n(CH4)*∆Ho(CH4) = 0.027*241.8 + 0.018*890.3 = 22.5 кДж.
271. Наука, изучающая возможность и направление самопроизвольного протекания химических реакций, называется химической термодинамикой. Химическая термодинамика позволяет вычислять количество энергии, необходимой для проведения реакции, может оценить глубину протекания процесса до достижения системой равновесного состояния, она исследует превращение энергии при химических реакциях и способность химических систем выполнять полезную работу.






