31. јбсолютные массы атомов и молекул не используютс€ в расчетах, т.к. имеют очень маленькие величины, с которыми работать не удобно. ¬ качестве единицы массы используетс€ атомна€ единица массы (а.е.м.), котора€ равна 1/12 массы изотопа углерода 12—. Ar(Na) = 23 а.е.м., Mr(NaOH) = 40 а.е.м., Mr(H2SO4) = 98 а.е.м.
32. јбсолютна€ масса атома (молекулы) равна отношению массы атома (молекулы) к 1/12 массы изотопа углерода 12—. ћасса одной молекулы кислорода = 32/6.02*1023 = 5.31*10-23 г. масса одного миллиарда молекул кислорода = 5.31*10-23 * 109 = 5.31*10-14 г.
33. оличество вещества измер€етс€ в химии в моль (не склон€етс€). ћоль составл€ют 6.02*1023 частиц. Ёто число называетс€ Ђчислом јвогадрої.
34. ќтносительной молекул€рной массой Mr вещества называют отношение массы молекулы вещества к 1/12 массы изотопа углерода 12—. ћоль - это такое количество вещества, которое содержит столько структурных единиц (атомов, молекул, ионов и т.п.), сколько их содержитс€ в 1/12 массы изотопа углерода 12—. ћол€рна€ масса - масса одного моль вещества. ћ(—аќ) = 56 г/моль, ћ(—а—ќ3) = 100 г/моль, ћ(—а(ќЌ)2) = 74 г/моль.
35. ћоль - единица измерени€ количества вещества в химии. ћоль - это такое количество вещества, которое содержит столько структурных единиц (атомов, молекул, ионов и т.п.), сколько их содержитс€ в 1/12 массы изотопа углерода 12—. ѕосто€нна€ јвогадро Na = 6.02*1023. ћол€рна€ масса - масса одного моль вещества. ћ(—ќ2) = 44 г/моль, ћ(HNќ3) = 63 г/моль, ћ(Ќ2SO4) = 98 г/моль, ћ(NH3) = 17 г/моль.
36. ѕростое вещество - группа атомов одного элемента, сложное вещество - группа атомов разных элементов. —ложные вещества - вода (Ќ2ќ - 18 г/моль), метан (—Ќ4 - 16 г/моль), аммиак (NH3 - 17 г/моль), серна€ кислота(H2SO4 - 98 г/моль). ѕростые вещества - хлор (Cl2 - 71 г/моль), кальций (Ca - 40 г/моль).
37. ќтносительна€ мол€рна€ масса молекулы равна сумме относительных мол€рных масс атомов, вход€щих в ее состав, умноженных на стехиометрические коэффициенты: что объ€сн€ет первый расчет. ћол€рна€ масса молекулы равна сумме мол€рных масс атомов, вход€щих в ее состав, умноженных на стехиометрические коэффициенты: что объ€сн€ет второй расчет.
38. ћоль - единица измерени€ количества вещества в химии. ћоль - это такое количество вещества, которое содержит столько структурных единиц (атомов, молекул, ионов и т.п.), сколько их содержитс€ в 1/12 массы изотопа углерода 12— - Na = 6.02*1023 (число јвогадро). ћол€рна€ масса - масса одного моль вещества. »з этих определений видно, что, несмотр€ на различную массу, один моль любого вещества содержит одинаковое число частиц. ќдин моль хлора Cl2 весит 75 г, один моль хлороводорода HCl весит 36,5 г, один моль хлороводородной кислоты HClO весит 52,5 г, но все эти количества содержат 6.02*1023 частиц.
39. ƒл€ азота а) масса одной молекулы m(N2) = M(N2)/Na = 28/6.02*1023 = 4.65*10-23г. б) ћолекул€рна€ масса азота = 28 а.е.м. в) ћол€рна€ масса азота = 28 г. г) ¬ одном грамме азота содержитс€ = 1/4.65*10-23 = 2.15*1022 молекул. ¬ 14 граммах азота содержитс€ = 14/4.65*10-23 = 3.01*1023 молекул. ¬ 28 граммах азота содержитс€ = 28/4.65*1023 = 6.02*1023 молекул.
|
|
|
40. ћасса одной молекулы воды = ћ(Ќ2ќ)/Na = 18/6.02*1023 = 2.99*10-23г. ¬ 0.1 грамме воды содержитс€ 0.1/2.99*10-23 = 3.34*1021 молекул. ≈сли отсчитывать по одной молекуле в секунду, то потребуетс€ 3.34*1021/(60*60*24*365) = 1.06*1014 лет.
41. ¬ одном килограмме иода содержитс€ m(I2)/M(I2)*Na = 1000*6.02*1023/254 = 2,37*1024 молекул. ≈сли отсчитывать по одной молекуле в секунду, то потребуетс€ 2,37*1024 /(60*60*24*365) = 7.52*1016 лет.
42. ѕосто€нна€ јвогадро (Na = 6.02*1023) равно количеству изотопов углерода 12—, содержащихс€ в 12 г. этого вещества. Ёто же количество частиц содержит любое вещество в количество 1 моль. ј. јвогадро €вл€етс€ одним из основоположников атомно-молекул€рного учени€ - ему принадлежит открытие известного газового закона: ¬ равных объемах различных газов при одинаковых температуре и давлении содержитс€ одинаковое число молекул.
43. „исло јвогадро Na = 6.0224*1023. „исло јвогадро было определено при помощи спектроскопических методов. „исло јвогадро равно количеству изотопов углерода 12—, содержащихс€ в 12 г. этого вещества.
44. а) 1 г. H2 n(H2) = m/M = 1/2 = 0.5 моль.
б) 4 кг. NaOH n(NaOH) = m/M = 4000/40 = 100 моль
в) 135 г. Al n(Al) = m/M = 135/27 = 5 моль
45. а) 0.8 кг. CuO n(CuO) = m/M = 800/80 = 10 моль.
б) 220 кг. Na2CO3 n(Na2CO3) = m/M = 220000/106 = 2075,5 моль
в) 56 г. KOH n(KOH) = m/M = 56/56 = 1 моль
46. а) 9.8 г. H2SO4 n(H2SO4) = m/M = 9.8/98 = 0.1 моль.
б) 98 г. H3PO4 n(H3PO4) = m/M = 98/98 = 1 моль
в) 160 г. O2 n(O2) = m/M = 160/32 = 5 моль
47. а) 1000 моль H2 m(H2) = n*M = 1000*2 = 2000 г.
б) 10 моль NaCl m(NaCl) = n*M = 10*58,5 = 585 г
в) 200 моль NH3 m(NH3) = n*M = 200*17 = 3400 г.
48. а) 5 моль Ar m(Ar) = n*M = 5*40 = 200 г.
б) 1 моль Fe m(Fe) = n*M = 1*56 = 56 г
в) 0.1 моль Au m(Au) = n*M = 0.1*197 = 19.7 г.
49. а) 50 моль Mg m(Mg) = n*M = 50*24 = 1200 г.
б) 10 моль Br2 m(Br2) = n*M = 10*160 = 1600 г
в) 0.1 моль KMnO4 m(KMnO4) = n*M = 0.1*158 = 15.8 г.
50. ѕоскольку мол€рна€ масса гидроксида натри€ меньше, чем у гидроксида кали€, то в 1 кг. NaOH содержитс€ больше молекул, чем в 1 кг. KOH. „исло частиц N = n*Na = (m*Na)/M
¬ 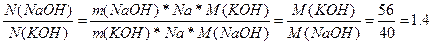 раза больше частиц в NaOH, чем в KOH.
раза больше частиц в NaOH, чем в KOH.
51. ѕоскольку мол€рна€ масса HNO3 меньше, чем у H2SO4, то в 1 кг. HNO3 содержитс€ больше молекул, чем в 1 кг. H2SO4. „исло частиц N = n*Na = (m*Na)/M
¬ 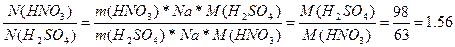 раза больше частиц в HNO3, чем в H2SO4
раза больше частиц в HNO3, чем в H2SO4
52. ѕусть m(H2) = m(N2) = m(CO2)=1.
“.к. количество вещества n = m/M, то n(H2): n(N2): n(CO2) = 1/2: 1/28: 1/44 = 1: 1/14: 1/22.
“.к. число молекул N = n*Na, то N(H2): N(N2): N(CO2) = 1*6.02*1023: 1/14*6.02*1023: 1/22*6.02*1023 = 1: 1/14: 1/22.
53. a) n(NaNO3) = 5 моль. n(N) = n(NaNO3) = 5 моль. m(N) = n*M = 5*14 = 70г.
б) n(NH4NO3) = 5 моль. n(N) = 2n(NH4NO3) = 10 моль. m(N) = n*M = 10*14 = 140г.
54. V(H2O) = 3 литра. —ледовательно, m(H2ќ) = 3 кг. n(H2O) = m/M = 3000/18 = 166.67 моль. ѕоскольку 1 моль любого вещества содержит одинаковое количество частиц, то 166.67 моль воды и 166.67 моль сероуглерода содержат равное количество частиц: n(CS2) = 166.67 моль. m(CS2) = n*M = 166.67*76 = 12666.7 г.
55. V(H2O) = 2 литра. —ледовательно, m(H2ќ) = 2 кг. n(H2O) = m/M = 2000/18 = 111.11 моль. ѕоскольку 1 моль любого вещества содержит 6.02*1023 частиц, то человек ежедневно потребл€ет 111.11*6.02*1023 = 6.69*1025 частиц.
|
|
|
56. m(Sx) = 4.25*10-22 г. —ера состоит из х = (4.25*10-22 * 6.02*1023)/Ar(S) = 255/32 = 8 атомов, где 6.02*1023 - число јвогадро, Ar(S) - относительна€ атомна€ масса серы.
57. m(–x) = 2.056*10-22 г. ‘осфор состоит из х = (2.056*10-22 * 6.02*1023)/Ar(–) = 124/31 = 4 атомов, где 6.02*1023 - число јвогадро, Ar(–) - относительна€ атомна€ масса фосфора.
58. m(ќx) = 8*10-23 г. ќзон состоит из х = (8*10-23 * 6.02*1023)/Ar(ќ) = 48/16 = 3 атомов, где 6.02*1023 - число јвогадро, Ar(ќ) - относительна€ атомна€ масса кислорода.
59. m(Na2CO3*x(H2O)) = 14.3 г., m(Na2CO3) = 5.3 г.
а) Ќайдем количество безводного карбоната натри€: n(Na2CO3) = m/M = 5.3/106 = 0.05 моль. б) ћасса воды равна m(H2O) = m(Na2CO3*x(H2O)) - m(Na2CO3) = 14.3 - 5.3 = 9 г.
в) оличество воды n(H2O) = m/M = 9 / 18 = 0.5 моль, следовательно, х = 0.5 / 0.05 = 10; ќтвет: Na2CO3*10(H2O).
60. m(FeSO4*x(H2O)) = 277.91 г., m(FeSO4) = 151,91 г.
а) Ќайдем количество безводного сульфата железа: n(FeSO4) = m/M = 151.91/152 = 1 моль. б) ћасса воды равна m(H2O) = m(FeSO4*x(H2O)) - m(FeSO4) = 277.91 - 151,91 = 126 г.
в) оличество воды n(H2O) = m/M = 126 / 18 = 7 моль, следовательно, х = 7 / 1 = 7; ќтвет: FeSO4*7(H2O).






