Содержание
Введение................................................................................................................. 4
1 Общие рекомендации по оформлению контрольной работы........................... 5
2 Методические указания по разделам контрольной работы.............................. 6
2.1 Основные понятия и стехиометрические законы химии…………………6
2.2 Основные классы неорганических соединений…...……………………..11
2.3 Химическая кинетика и равновесие………………………………………17
2.4 Способы выражения состава растворов. Коллигативные свойства растворов………………...…………………………………………………26
2.5 Электролитическая диссоциация, ионное произведение воды, произведение растворимости……………………………………….…….32
2.6 Буферные растворы………….……………………………………………..40
2.7 Гидролиз солей……………………………………………………………..45
2.8 Жесткость воды…………………………………………………………….51
2.9 Коллоидно-дисперсные системы………………………………………….55
2.10 Окислительно-восстановительные реакции и электрохимические системы……………………..……………………………………………..60
3 Варианты заданий…………………………………………………………………...68
3.1 Варианты заданий при наличии в учебном плане одной контрольной работы……………………………………………………………………...68
3.2 Варианты заданий при наличии в учебном плане двух контрольных работ……………………………………………………………………….70
3.3 Условия контрольных заданий…………………………………………73
4 Программа дисциплины…………………………………………………………….92
5 Список рекомендуемой литературы……………………………….………………96
Заключение............................................................................................................97
Использованная литература.................................................................................98
Введение
Настоящие методические указания составлены в соответствии c рабочей программой, полностью соответствующей ФГОС ВПО для бакалавров по направлению подготовки 270800 «Строительство». Они учитывают специфику данного направления подготовки и различный первоначальный уровень химических знаний студентов, а потому являются актуальными.
Целью данной методической разработки является повышение эффективности развития теоретических умений у студентов, что способствует формированию общекультурных и профессиональных компетенций, предусмотренных в ФГОС ВПО для указанного направления подготовки бакалавров.
Эта цель достигается существенной методической и дидактической поддержкой самостоятельной работы студентов при выполнении заданий контрольной работы.
Методический материал включает общие рекомендации по оформлению контрольной работы, краткие теоретические пояснения и примеры решения типовых задач по каждому разделу контрольной работы, список рекомендуемой литературы.
Общие рекомендации по оформлению
Контрольная работа выполняется в тонкой тетради в клеточку с отведенными полями.
На обложке указывается вид работы, номер варианта, факультет, курс, специальность, номер группы, фамилия, имя и отчество студента (полностью), номер зачетки (шифр).
Номер варианта определяется по двум последним цифрам зачетки (смотрите пояснения в таблице 1.1).
Таблица 1.1 – Определение номера варианта
| Две последние цифры зачетки | Номер варианта |
Если в учебном плане предусмотрена только одна контрольная работа, то номера контрольных заданий берутся из таблицы 3.1.
При наличии в учебном плане двух контрольных работ номера контрольных заданий определяются в соответствии с таблицей 3.2.
При оформлении работы указывается номер задания в соответствии с таблицей вариантов заданий (таблица 3.1 или таблица 3.2) и приводится его полный текст, затем следует решение со всеми необходимыми расчетами, уравнениями реакций и текстовыми пояснениями.
Работа сдается для регистрации в методический кабинет до начала экзаменационной сессии. Если контрольная работа содержит ошибки, их нужно исправить в соответствии с указаниями преподавателя, проверявшего работу, и выполнить работу над ошибками в той же тетради.
Студент, не предоставивший удовлетворительно выполненную контрольную работу, к экзамену не допускается.
Методические указания по разделам контрольной работы
Основные понятия и стехиометрические законы химии
Прежде чем приступать к решению задач этого раздела, необходимо четко уяснить понятия: относительная атомная и молекулярная массы, формульная масса, моль, постоянная Авогадро, молярная масса, молярный объем, эквивалент, число эквивалентности, количество вещества эквивалента, молярная масса эквивалента. Требуются также знания уравнения состояния идеального газа (уравнение Клапейрона-Менделеева), закона Авогадро и следствий из него, закона Дальтона для парциальных давлений газов, закона эквивалентов. Необходимо уметь выражать связь между массой, молярной массой и количеством вещества, а также связь между молярной массой эквивалента, количеством вещества эквивалента, массой и молярной массой вещества. Требуется научиться применять закон эквивалентов для нахождения масс и молярных масс веществ, участвующих или образующихся в реакции, уметь определять эквивалент и молярную массу эквивалента вещества в конкретной реакции.
Так как понятие эквивалента за последние десятилетия претерпело существенное изменение, для его усвоения следует пользоваться современными изданиями учебников.
Химическим эквивалентом называется реальная или условная частица вещества, которая может замещать, присоединять, высвобождать или быть каким-либо другим способом эквивалентна (равноценна) одному иону водорода в кислотно-основных и ионообменных реакциях, либо одному электрону в окислительно-восстановительных реакциях.
Реальные частицы – это атомы, ионы, молекулы и так далее, а условные частицы – это, например, 1/2 Н2SO4, 1/4 C, 1/3 Fe3+.
В общем случае эквивалент любого вещества X может быть записан в виде 1/z(X), где z – число эквивалентности, или эквивалентное число, которое всегда ³ 1. Оно показывает, сколько эквивалентов содержится в одной формульной единице вещества.
Для данного вещества z находится по конкретной реакции. В окислительно-восстановительных процессах z определяется числом электронов, принятых или отданных одной формульной единицей вещества. Рассмотрим окислительно-восстановительную реакцию
S + O2 = SO2
S0 - 4e- = S+4
O2 + 4e- = 2O –2.
Формульная единица серы отдает 4 электрона, а одна формульная единица молекулярного кислорода принимает 4 электрона. Следовательно, z для серы и для кислорода равно четырем. Поэтому эквивалент серы в приведенной реакции - условная частица ¼ S, а эквивалент кислорода – ¼ О2.
В ионообменных процессах величина z определяется стехиометрией реакции, причем для одного и того же вещества, в зависимости от реакции, эквивалентное число может иметь различное значение.
В качестве примера рассмотрим две реакции:
H2SO4 + NaOH = NaHSO4 + H2O;
H2SO4 + 2 NaOH = Na2SO4 + 2 H2O.
Эквивалентом гидроксида натрия является реальная частица NaOH, для нее z = 1. В соответствии с уравнением первой реакции, один эквивалент NaOH взаимодействует с одной частицей H2SO4. Поэтому число эквивалентности для серной кислоты в этой реакции равно 1, а эквивалентом будет являться реальная частица H2SO4.
Во второй реакции два эквивалента гидроксида натрия реагируют с одной частицей серной кислоты, тогда один эквивалент NaOH взаимодействует с условной частицей 1/2 H2SO4, которая и будет являться эквивалентом серной кислоты во второй реакции. Таких частиц в формульной единице H2SO4 две, поэтому для серной кислоты в данной реакции z = 2.
Когда речь не идет о конкретной реакции, при определении z для сложных веществ можно воспользоваться следующими правилами:
- для оксидов z равно числу атомов элемента, умноженному на степень окисления элемента;
- для кислот z равно основности кислоты;
- для оснований z равно кислотности основания;
- для солей z равно числу катионов металла, умноженному на заряд катиона.
Количество вещества эквивалента обозначается n(1/z(X)). Единица измерения – моль. 1 Моль эквивалента вещества содержит 6,022×1023 эквивалентов.
Молярная масса эквивалента М(1/z(X))– это масса 1 моль эквивалентавещества 1/z(X), измеряется в г/моль.
Связь между молярной массой эквивалента, количеством вещества эквивалента, массой и молярной массой вещества выражается соотношениями
 (2.1.1)
(2.1.1)
Например, молярная масса эквивалента серной кислоты может быть вычислена как M(1/2H2SO4) = M(H2SO4):2 = 98:2 = 49 (г/моль).
Закон эквивалентов гласит: «Эквивалентные количества всех веществ, участвующих в реакции, одинаковы».
n (1/z (X1)) = n (1/z (X2))(2.1.2)
Например, для реакции H2SO4 + 2NaOH = Na2SO4 + 2H2O закон запишется в виде равенств
n(1/2 H2SO4) = n(NaOH) = n(1/2 Na2SO4) = n(H2O)
Из закона эквивалентов следует, что массы реагирующих друг с другом веществ, а также массы продуктов реакции относятся друг к другу как молярные массы эквивалентов этих веществ.
В общем виде для двух компонентов реакции Х1 и Х2 справедлива формула
 (2.1.3)
(2.1.3)
Если один из компонентов реакции, допустим Х1, находится в газообразном состоянии, то для него справедливы соотношения:
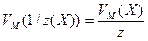 ;
; 
где V(X) – объем газообразного вещества X;
VM(1/z(X)) – объем, занимаемый одним моль эквивалента вещества Х;
VM(X) – молярный объем газообразного вещества Х, равный при нормальных условиях 22,4 л/моль.
Тогда равенство (2.1.3) преобразуется к виду
 (2.1.4)
(2.1.4)
Приведем другие формулы, использующиеся при решении задач этого раздела.
Молярная масса вещества М:
 , (2.1.5)
, (2.1.5)
где m – масса вещества, г;
n - количество вещества, моль.
Единица измерениямолярной массы – г/моль.
Число Авогадро – число частиц, содержащихся в 1 моль.
NA= 6,02∙1023 моль -1.
Молярный объем VM:
VM = V: n, (2.1.6)
где V - объем газа;
n - количество вещества газа, моль.
При нормальных условиях молярный объем любого газа равен 22,4 л/моль (следствие из закона Авогадро).
Отношение масс равных объемов газов при одинаковых условиях равно отношению их молярных масс (следствие из закона Авогадро):
 (2.1.7)
(2.1.7)
Величина  называется относительной плотностью первого газа по второму.
называется относительной плотностью первого газа по второму.
Закон Дальтона для парциальных давлений не реагирующих друг с другом газов:
Робщ.=Р1+Р2+…+Рn, (2.1.8)
где Робщ – общее давление смеси не реагирующих газов;
Р1, Р2, … Рn – парциальные давления компонентов смеси.
Объединенный газовый закон:
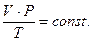 (2.1.9)
(2.1.9)
Уравнение состояния идеального газа Клапейрона – Менделеева связывает между собой такие величины, как молярная масса газа М, его масса m, давление Р, объем V и температура Т:
 , (2.1.10)
, (2.1.10)
где R – универсальная газовая постоянная, равная 8,314  .
.
Пример 1
Определить молярную массу эквивалента Fe2(SO4)3 в реакции
Fe2(SO4)3 + 6 NaOH = 2 Fe(OH)3 + 3 Na2SO4.
Решение
Одна формульная единица гидроксида натрия взаимодействует с одним ионом водорода, поэтому эквивалент щелочи есть реальная частица NaOH. По стехиометрии реакции на одну частицу NaOH (эквивалент) приходится 1/6частицы Fe2(SO4)3, поэтому эквивалентом сульфата железа (III) в данной реакции будет условная частица 1/6 Fe2(SO4)3.
Молярная масса эквивалента сульфата железа (III) в данной реакции может быть вычислена по формуле
M(1/6 Fe2(SO4)3) = M(Fe2(SO4)3) / 6 = 400 / 6 = 66,7 (г/моль).
Пример 2
Хлорид никеля массой 2,918 г взаимодействует с 1,8 г гидроксида натрия, молярная масса эквивалента которого равна 40 г/моль. Вычислить молярную массу эквивалента хлорида никеля.
Решение
В соответствии с законом эквивалентов
m(хлорида) / m(NaOH) = M(1/z хлорида) / M(1/1 NaOH).
Отсюда
M(1/z хлорида) = 2,918 × 40 / 1,8 = 64,8 (г/моль)
Пример 3
При сжигании 2,96 г металла было получено 4,91 г его оксида. Определить молярную массу эквивалента металла.
Решение
Найдем массу кислорода, израсходованного при горении металла. В соответствии с законом сохранения массы веществ
m(O2) = m(оксида) – m(металла) = 4,91 – 2,96 = 1,95 (г).
При окислении металлов до оксидов газообразным кислородом происходит процесс: О20 + 4е- = 2О–2. Молекула кислорода принимает 4 электрона, следовательно, z = 4. На один электрон приходится 1/4 О2, поэтому эквивалент молекулярного кислорода 1/4 O2, а молярная масса эквивалента равна 8 г/моль.
По закону эквивалентов m(O2) / m(Me) = M(1/4 O2) / M(1/z Me);
Отсюда выразим M(1/z Me) и подставим численные значения величин:
M(1/z Me) = 2,96 × 8 / 1,95 = 12,1 (г/моль)
Пример 4
Некоторый газ, имеющий плотность по воздуху, равную 0,965, был собран в сосуд емкостью 2 л методом вытеснения воды и находится в нем при температуре 25 оС и давлении 99,8 кПа. Определите массу газа в сосуде, если давление паров воды составляет 3,17 кПа.
Решение
Так как газ был собран в сосуд методом вытеснения воды, то он содержит пары воды. В соответствии с законом Дальтона для смеси не взаимодействующих газов, общее давление газовой смеси складывается из парциальных давлений отдельных ее компонентов. Найдем давление неизвестного газа.
Ргаза=Рсмеси–Рпара воды= 99,8 кПа– 3,17 кПа= 96,63 кПа.
Зная относительную плотность газа по воздуху, рассчитаем его молярную массу в соответствии со следствием из закона Авогадро.
Мгаза=Dвозд.×Мвозд= 0,965 × 29 г/моль» 28 г/моль.
Выразим массу газа m из уравнения Клапейрона-Менделеева и подставим в полученное выражение все величины в единицах системы СИ.
 ;
;

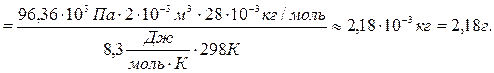
Итак, масса газа в сосуде равна 2,18 г.
Основные классы неорганических соединений
По функциональным признакам к основным классам неорганических соединений принято относить оксиды, гидроксиды (основные, кислотные, амфотерные) и соли, которые можно рассматривать как продукты взаимодействия различных по кислотно-основным свойствам оксидов и гидроксидов. Включение именно этих классов соединений в группу основных неорганических соединений обусловлено химическими особенностями земной атмосферы, где главным окислителем является кислород, а также тем, что самой распространенной жидкостью на Земле является вода.
Генетическая связь между основными классами неорганических соединений показана на рисунке 2.2.1.
Сложные неорганические вещества по составу делятся на бинарные (двухэлементные), например оксиды, галогениды, сульфиды, гидриды, нитриды, карбиды, и многоэлементные соединения.
Оксиды – это бинарные соединения элементов с кислородом, в которых он проявляет степень окисления –2. Бинарные соединения с фтором, где кислород проявляет положительную степень окисления, а также пероксиды (степень окисления –1), супероксиды (степень окисления –1/2), озониды (степень окисления –1/3) оксидами не являются.
По функциональным признакам оксиды делятся на солеобразующие (при взаимодействии с кислотами или основаниями дают соли) и несолеобразующие, которые не образуют солей, им не соответствуют гидроксиды с той же степенью окисления элемента, что и в оксиде. Несолеобразующие оксиды могут вступать с кислотами или основаниями только в окислительно-восстановительное взаимодействие. Примером таких оксидов служат N2O, NO, CO, SiO, OsO4 SO, SeO, TeO и другие.
Солеобразующие оксиды подразделяются на основные, кислотные (ангидриды кислот) и амфотерные.
К основным оксидам относятся оксиды щелочных и щелочноземельных металлов, MgO, CuO (основные свойства преобладают), CdO, HgO, VO, CrO, MnO, FeO, NiO, CoO, Bi2O3 и другие. Основные оксиды взаимодействуют с кислотами и кислотными оксидами с образованием солей. Например,
CaO + 2HCl = CaCl2 + H2O; SO3 + CaO = CaSO4.
Они генетически связаны с металлами, имеющими небольшую степень окисления (+1, +2, редко +3). Непосредственно с водой взаимодействуют оксиды щелочных и щелочноземельных металлов, частично MgO. При этом образуются основные гидроксиды.
BaO+H2O=Ba(OH)2

| +H2O | Растворимые основные гид-роксиды (ще-лочи) | С о л и | |||||||
О2
|  Основные оксиды Основные оксиды
| 
|  
|  
| ||||||
Металлы
| 
| 
|  С о л и С о л и
| +щелочь | Малораство-римые основные гид-роксиды | 
| ||||

|  Амфотерные оксиды Амфотерные оксиды
|
 +щелочь +щелочь
| 
| |||||||
Неметаллы

|  
| Амфотерные гидроксиды | ||||||||
| O2 |  Кислотные оксиды Кислотные оксиды
| +более сильная |  
| |||||||
| кислота +H2O | Кислотные гидроксиды (кислородсо-держащие кислоты) |
Рисунок 2.2.1 – Генетическая связь между основными классами неорганических соединений
Кислотные оксиды взаимодействуют с основаниями и основными оксидами с образованием солей.
Ca(OH)2 + CO2 = CaCO3 ¯ + H2O
Многие кислотные оксиды, за небольшим исключением (SiO2, TeO2, TeO3, MoO3, WO3 и другие), непосредственно взаимодействуют с водой, образуя кислородсодержащие кислоты.
P2O5+3H2O=2H3PO4
Кислотные оксиды генетически связаны с неметаллами, а также металлами в высоких степенях окисления: +4, +7. К кислотным оксидам относятся CO2, SiO2, SO2, SO3, P2O5, N2O3, NO2, N2O5, B2O3, CrO3, Mn2O7 и другие.
Амфотерные оксиды взаимодействуют как с кислотами, так и с основаниями с образованием солей.
ZnO+ 2HCl = ZnCl2 + H2O;
ZnO + 2NaOH +H2O = Na2[Zn(OH)4].
Эти оксиды непосредственно с водой не взаимодействуют, генетически связаны с некоторыми металлами в степени окисления +2 и почти со всеми металлами в степенях окисления +3, +4. К амфотерным оксидам относятся BeO, ZnO, PbO, SnO, Al2O3, Cr2O3, SnO2, PbO2, Sb2O3 и другиие.
Как показывают приведенные примеры, с повышением степени окисления металла основные свойства их оксидов ослабевают, а кислотные усиливаются.
Существуют так называемые смешанные оксиды. Некоторые из них относятся к оксидам лишь формально. Их следует рассматривать как соли металла и кислородсодержащей кислоты, в состав которой входит тот же металл, но в более высокой степени окисления. Например, Pb2O3 – свинцовая соль метасвинцовой кислоты (H2PbO3). Однако в кристаллической решетке Fe3O4 не содержится групп атомов, соответствующих кислотному остатку, поэтому данное соединение рассматривают как смешанный оксид Fe (II) и Fe(III).
Двойные оксиды составов (М΄,М2) О4 и(М2΄,М) О4, где М΄ – металл в степени окисления +2, называются шпинелями. Например, MgAl2O4 – тетраоксид диалюминия-магния называют также алюмомагниевой шпинелью.
Названия оксидов образуются следующим образом:
˗ слово «оксид» и название элемента в родительном падеже с указанием в скобках римской цифрой его степени окисления (если элемент может проявлять несколько степеней окисления);
˗ стехиометрические соотношения между элементами указываются при помощи греческих умножающих префиксов, присоединяемых без дефиса к названиям элементов (если в формуле свыше 12 атомов одного вида, то вместо префиксов используются цифры).
Например, СО2 – оксид углерода (IV) или диоксид углерода, N2O – оксид азота (I) или оксид диазота, Fe3O4 – оксид дижелеза (III)-железа (II) или тетраоксид трижелеза, W20O58 – 58 – оксид 20 – вольфрама.
Солеобразующим оксидам соответствуют гидроксиды – соединения, содержащие гидроксильные группы ОН. По кислотно-основным свойствам гидроксиды подразделяются на основные, кислотные и амфотерные.
Основные гидроксиды диссоциируют в водных растворах с образованием в качестве анионов только ОН-. Они подразделяются на малорастворимые в воде основания и хорошо растворимые основания или щелочи. Важнейшее химическое свойство основных гидроксидов – способность взаимодействовать с кислотами и кислотными оксидами с образованием солей:
NaOH + HCl = NaCl + H2O
Кислотные гидроксиды – это кислородсодержащие кислоты, в состав которых входят гидроксильные группы. Далее приводятся структурные формулы трех кислородсодержащих кислот:
H2SO4 HNO3 H2CO3



Кислотные гидроксиды диссоциируют в воде с образованием в качестве катионов только ионов водорода Н+. Кислотные гидроксиды взаимодействуют с основаниями и основными оксидами с образованием солей.
Следует отметить, что понятие кислоты шире, чем понятие кислотного гидроксида, так как существуют и бескислородные кислоты, например, HCl, HF, H2S, HCN и другие.
Амфотерные гидроксиды могут взаимодействовать как с кислотами, так и со щелочами с образованием солей.
Zn(OH)2 + H2SO4 = ZnSO4 + 2H2O;
Zn(OH)2 + 2NaOH = Na2[Zn(OH)4].
Названия основных и амфотерных гидроксидов состоят из слова «гидроксид» и названия элемента в родительном падеже с указанием в скобках римскими цифрами его степени окисления (если данный элемент может проявлять несколько степеней окисления).
В номенклатуре кислотных гидроксидов (кислот) используются как тривиальные, так и систематические названия. Последние полностью отражают состав соединения и даются по правилам составления названий комплексных соединений. Систематические названия рекомендуется давать лишь малораспространенным кислотам, образованным элементами с переменной степенью окисления. Например, H6TeO6 – гексаоксотеллурат (VI) водорода. Однако серную кислоту вовсе не требуется называть тетраоксосульфат (VI) водорода, за ней сохраняется традиционное название.
При взаимодействии между собой гидроксидов и оксидов, с различными кислотно-основными свойствами, образуются соли. Соли по составу подразделяются на простые, двойные, смешанные и комплексные.
Двойные соли образованы двумя различными катионами и одним анионом. Например, KAl(SO4)2 – сульфат алюминия-калия.
Смешанные соли образованы несколькими различными анионами (кислотными остатками) и одним катионом. Например, Ca(ClO)Cl – хлорид-гипохлорит кальция или хлорная известь.
Комплексные соли содержат в своем составе сложные комплексные ионы, которые в химических реакциях, процессах растворения, в структуре кристалла ведут себя как самостоятельные единицы. Например, K4[Fe(CN)6] гексацианоферрат (II) калия диссоциирует в воде на ионы в соответствии с уравнением
K4[Fe(CN)6]  4K+ + [Fe(CN)6] 4-.
4K+ + [Fe(CN)6] 4-.
Комплексный анион практически не диссоциирует в водном растворе, поэтому Fe2+ не обнаруживается качественными реакциями.
Простые соли по характеру замещения подразделяются на средние (нормальные), кислые и основные.
Средние соли, например, CuSO4, Na2CO3 и другие, являются продуктами полного замещения ионов водорода в кислоте на другие катионы или продуктами полного замещения гидроксильных групп в основании на кислотные остатки.
Перечислим некоторые основные способы образования средних солей:
- взаимодействие металлов с кислотами
Zn + 2HCl = ZnCl2 + H2;
- взаимодействие металлов, оксиды которых амфотерны, со щелочами
Zn + 2NaOH + 2H2O = Na2[Zn(OH)4] + H2;
- взаимодействие основания с кислотой
NaOH + HCl = NaCl + H2O;
- взаимодействие основания с кислотным оксидом
Ca(OH)2 + CO2 = CaCO3¯ + H2O;
- взаимодействие кислоты с основным оксидом
CaO + 2HCl = CaCl2 + H2O;
- взаимодействие кислоты с солью (более сильная кислота вытесняет более слабую, летучую, разлагающуюся или выпадающую в осадок кислоту из ее соли)
2CH3COONa + H2SO4 = 2CH3COOH + Na2SO4;
- взаимодействие растворимого основания с солью
FeCl3 + 3NaOH = Fe(OH)3¯ + 3NaCl;
- взаимодействие между солями
NaCl + AgNO3 = AgCl¯ + NaNO3;
- взаимодействие солей с металлами
CuSO4 + Zn = Cu¯ + ZnSO4;
- взаимодействие основных и кислотных оксидов
SO3 + CaO = CaSO4;
- взаимодействие металлов с неметаллами
2Fe + 3Cl2 = 2FeCl3.
Кислые соли можно рассматривать как продукты неполного замещения ионов водорода в двух- или более основной кислоте на другие катионы. Кислые соли получаются при взаимодействии кислоты или кислотного оксида с недостатком основания, либо взаимодействием средней соли с кислотой или кислотным оксидом.
H2SO4 + NaOH = NaHSO4 + H2O; CO2 + KOH = KHCO3;
Ca3(PO4)2 + H3PO4 = 3CaHPO4; CaCO3 + CO2 + H2O = Ca(HCO3)2.
Так как в анионе кислой соли содержится подвижный водород, то она частично сохраняет свойства кислоты и может вступать в реакцию нейтрализации с основаниями.
NaHSO4 + NaOH = Na2SO4 + H2O.
Основные соли можно рассматривать как продукты неполного замещения ОН – групп в многокислотных основаниях на кислотные остатки. Эти соли получаются при взаимодействии основания с недостатком кислоты или средней соли с основанием.
Mg(OH)2 + HCl = MgOHCl + H2O;
CoCl2 + NaOH = CoOHCl¯ + NaCl.
Так как в состав основных солей входят гидроксильные группы, то эти соли могут взаимодействовать с кислотами с образованием средних солей. Таким образом, основные соли частично сохраняют свойства оснований.
MgOHCl + HCl = MgCl2 + H2O
Систематические названия солей связаны с систематическими названиями соответствующих кислот. Традиционные названия солей кислородсодержащих кислот составляются из названия аниона в именительном падеже и катиона в родительном падеже. Название аниона включает корень русского или латинского названия кислотообразующего элемента с добавлением суффикса, соответствующего степени окисления элемента. При этом возможны следующие случаи:
- если кислотообразующий элемент имеет только одну степень окисления, то добавляется суффикс –ат (Na2CO3 – карбонат натрия);
- если кислотообразующий элемент имеет две степени окисления, то при высшей из них к корню добавляется суффикс –ат, а при низшей степени окисления добавляется суффикс – ит (CaSO4 – сульфат кальция, Na2SO3 – cульфит натрия);
- если имеются анионы, отвечающие четырем степеням окисления кислотообразующего элемента, то для высшей степени окисления используется приставка пер- и суффикс -ат (КCl+7O4 – перхлорат калия), затем суффикс –ат (KCl+5O3 – хлорат калия), суффикс –ит (KCl+3O2 – хлорит калия) и для наименьшей степени окисления – приставка гипо- и суффикс –ит (KCl+1O – гипохлорит калия);
- в названиях анионов солей бескислородных кислот используется суффикс –ид (K2S – сульфид калия);
- при построении традиционных названий кислых солей к названию аниона средней соли добавляется приставка гидро- и числовая греческая приставка, если число атомов водорода в анионе больше одного, например, Ca(H2PO4)2 – дигидрофосфат кальция, Ca(HCO3)2 – гидрокарбонат кальция;
- традиционные названия основных солей формируются при помощи приставки гидроксо- и при необходимости соответствующей числительной приставки, например, Al(OH)2NO3 – нитрат дигидроксоалюминия. Обратите внимание, что сначала называется анион в именительном падеже, а затем сложный катион в родительном падеже.








