Приклад 1. Розрахувати розчинність CaSO4 у воді, в розчині KNO3 з молярною концентрацією калій нітрату 0,01 моль/дм 3 і в розчині Mg(NO3)2 з молярною концентрацією магній нітрату 0,01 моль/дм 3.
Дано:
c (KNO3) = 0,01 моль/дм 3 ДP (CaSO4) =2,37∙10–5
 c (Mg(NO3)2) = 0,01 моль/дм 3
c (Mg(NO3)2) = 0,01 моль/дм 3
S (CaSO4) –?
Розв’язування
Позначимо розчинність CaSO4 у воді через х. Так як CaSO4 при розчиненні дає по одному йону Ca2+ і  ,то
,то
[Ca2+] = [  ] = [CaSO4] = x
] = [CaSO4] = x
ДР(CaSO4) = [Ca2+]·[  ] = x · x = 2,37∙10–5
] = x · x = 2,37∙10–5
x 2= 2,37·10–5; x =  = 4,868·10–3 моль/дм 3
= 4,868·10–3 моль/дм 3
Знайдемо розчинність CaSO4 у розчині KNO3 з молярною концентрацією речовини 0,01 моль/дм 3. Йонна сила розчину буде визначатися концентрацією і зарядами всіх присутніх у розчині йонів:
μ = ½([K+]·12 + [ 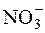 ]·12 + [Ca2+]·22 + [
]·12 + [Ca2+]·22 + [  ]·22) = ½(0,01·12 +
]·22) = ½(0,01·12 +
+ 0,01·12 + 0,004868·22 + 0,004868·22) = ½(0,01 + 0,01 + 0,0195 +
+ 0,0195) = 0,0295
Так як у таблиці немає йонної сили розчину, що дорівнює 0,0295, то величину коефіцієнтів активності  =
=  знаходять методом інтерполяції:
знаходять методом інтерполяції:
 =
=  = 0,55–0,018 = 0,532 або приблизно 0,53
= 0,55–0,018 = 0,532 або приблизно 0,53
ДР (CaSO4) = [Ca2+]·[  ]∙
]∙  ∙
∙  = x ∙ x ∙0,532 = 2,37∙10–5
= x ∙ x ∙0,532 = 2,37∙10–5
x 2∙0,532 = 2,37∙10–5
x =  = 9,2∙10–3 моль/дм 3
= 9,2∙10–3 моль/дм 3
Знайдемо розчинність СaSO4 у розчині Mg(NO3)2 з молярною концентрацією речовини 0,01 моль/дм 3.
У цьому випадку йонна сила розчину становить:
μ = ½([Mg2+]∙22 + 2[ 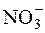 ]·12 + [Ca2+]·22 + [
]·12 + [Ca2+]·22 + [  ]·22) =
]·22) =
= ½(0,01·4 + 2·0,01 + 0,004868·4 + 0,004868·4) = 0,0495 ≈ 0,05
Отже,  =
=  = 0,45.
= 0,45.
ДР (CaSO4) = [Ca2+]∙[  ]∙
]∙  ∙
∙  = x ∙ x ∙0,452 = 2,37∙10–5
= x ∙ x ∙0,452 = 2,37∙10–5
x 2∙0,452 = 2,37∙10–5
x =  = 1,082∙10–2 моль/дм 3
= 1,082∙10–2 моль/дм 3
З цих розрахунків випливає, що розчинність кальцій сульфату в розчинні калій нітрату (с =0,01 моль/дм 3) приблизно в 1,9 рази більша від розчинності у чистій воді, а розчинність CaSO4 у розчині Mg(NO3)2 (с =0,01 моль/дм 3) приблизно в 2,2 рази більша, ніж у чистій воді.
Приклад 2. Обчислити інтервал значень p H розчину для кількісного розділення йонів Fe3+ і Mg2+ шляхом осадження їх у формі гідроксидів.
Розв’язування
Спочатку розраховується значення p H розчину, при якому буде досягнута повнота посадження йонів Fe3+ у вигляді Fe(OH)3. Критерієм повноти осадження буде [Fe3+ ]£1,0∙10–6 моль/дм 3.
Виходячи з добутку розчинності
ДР (Fe(OH)3) = [Fe3+]∙[OH–]3 = 6,3∙10–38,
знаходять концентрацію йонів ОН–, при якій буде досягнута повнота осадження йонів Fe3+:
 моль/дм 3
моль/дм 3
Звідси
 моль/дм 3
моль/дм 3
рH = –lg[H+] = –lg(2,5∙10–4) = 3,6
Отже, для повноти осадження йонів Fe3+ у формі Fe(OH)3 необхідно підтримувати в розчинні p H 3,6.
Йони Mg2+, будуть залишатися в розчинні до тих пір, доки не буде досягнуто добуток розчинності Mg(OH)2, тобто умовою збереження йонів Mg2+ у розчинні буде
[Mg2+]∙[OH–]2 < ДР (Mg(OH)2); ДР(Mg(OH)2) = 6,0∙10–10
Звідси
[OH–]£  £
£  моль/дм 3
моль/дм 3
Якщо у вихідному розчині [Mg2+]=0,1 моль/дм 3, то умовою невипадіння в осад Mg(OH)2 буде
[OH–]£  моль/дм 3; [OH–]£7,8∙10–5 моль/дм 3,
моль/дм 3; [OH–]£7,8∙10–5 моль/дм 3,
а якщо [Mg2+]=1,0∙10–2 моль/дм 3, то
[OH–]£  моль/дм 3; [OH–]£2,5∙10–4 моль/дм 3,
моль/дм 3; [OH–]£2,5∙10–4 моль/дм 3,
Якщо концентрація йонів Mg2+ у вихідному розчині становить 0,1 моль/дм 3, то для збереження цих йонів у розчині [OH–]£7,8∙10–5 моль/дм 3, що відповідає
[H+]=  =1,3·10–10 моль/дм 3, або p H£9,9.
=1,3·10–10 моль/дм 3, або p H£9,9.
Таким чином, якщо у розчині буде підтримуватися значення p H не менше, ніж 3,6 і не більше, ніж 9,9, то Fe3+ повністю буде в осаді у вигляді Fe(OH)3, а йони Mg2+ повністю залишаться в розчині. Цю умову можна записати у вигляді формули:
3,6£ p H£9,9.
Приклад 3. Який об’єм розчину аргентум нітрату з масовою часткою AgNO3 2% необхідно взяти для осадження йонів Cl– із наважки CaCl2∙6H2O масою 0,4382 г?
Дано:
m (CaCl2∙6H2O) = 0,4382 г
 w (AgNO3) = 2%
w (AgNO3) = 2%
V (AgNO3(p)) –?
Розв’язування
Обчислення виконуються наближено, тому числа, які беруться для розрахунків, слід округлити.
Визначається маса аргентум нітрату, необхідна для осадження йонів Cl–:

Далі визначається об’єм розчину AgNO3 (w (AgNO3)=2%), у якому міститься розрахована маса AgNO3. Густину розчину можна прийняти за одиницю.

Приклад 4. Обчислити втрату маси і відносну похибку за рахунок розчинності осаду CaC2O4·H2O, якщо до 20 см 3 розчину CaCl2 з молярною концентрацією речовини CaCl2 0,1 моль/дм 3 додали 30 см 3 розчину (NH4)2C2O4 з молярною концентрацією речовини амоній оксалату 0,1 моль/дм 3.
Дано:
V (CaCl2(p)) = 20 см 3 ДР (СаС2О4) = 2,3∙10–9
с (CaCl2) = 0,1 моль/дм 3 М (СаС2О4∙Н2О) = 146,12 г/моль
V ((NH4)2C2O4(p)) = 30 см 3
с ((NH4)2C2O4) = 0,1 моль/дм 3
 m (CaC2O4·H2O) –?
m (CaC2O4·H2O) –?
η –?
Розв’язування
Після зливання розчинів кальцій хлориду і амоній оксалату об’єм утвореного розчину становить: 20 см 3+30 см 3=50 см 3. Надлишок розчину осаджувача складає 10 см 3. Концентрація оксалат-іонів у отриманому розчині дорівнює:
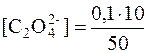 = 0,02 моль/дм 3
= 0,02 моль/дм 3
Позначимо через х розчинність СаС2О4∙Н2О. Тоді [Са2+]= х моль/дм3, [  ]=(0,02+ х) моль/дм 3. Виходячи із значення ДР (СаС2О4),
]=(0,02+ х) моль/дм 3. Виходячи із значення ДР (СаС2О4),
[Са2+]·[  ] = х ∙(0,02+ х) = 2,3∙10–9
] = х ∙(0,02+ х) = 2,3∙10–9
Так як х « 0,02, то (0,02+ х) ≈ 0,02. Отже, х ∙0,02 = 2,3∙10–9. Звідси:
 = 1,15∙10–7 моль/дм 3
= 1,15∙10–7 моль/дм 3
Розчинність СаС2О4 дорівнює 1,15∙10–7 моль/дм 3. Втрати через розчинність кальцій оксалату у розчині об’ємом 50 см 3 становитимуть:
 = 8,4∙10–7 г.
= 8,4∙10–7 г.
Маса осаду СаС2О4∙Н2О, отриманого при зливанні розчинів кальцій хлориду і амоній оксалату становить:
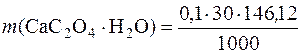 = 0,4383 г.
= 0,4383 г.
Тоді η =  = 1,9·10–4%.
= 1,9·10–4%.
Приклад 5. Обчислити масову частку втрат магній гідроксиду за рахунок розчинності осаду Mg(OH)2 масою 0,2 г при промиванні водою об’ємом 250 см 3. Визначити концентрацію амоніаку у промивній рідині, щоб втрати при промиванні осаду таким же об’ємом склали не більше 0,1%.
Дано:
m (Mg(OH)2) = 0,2 г ДР (Mg(OH)2) = 6,0·10–10
V (H2O) = 250 см 3 M (Mg(OH)2) = 58,32 г/моль
 V (NH3(р)) = 250 см 3 Kд (NH3·H2O) = 1,76·10–5
V (NH3(р)) = 250 см 3 Kд (NH3·H2O) = 1,76·10–5
w (Mg(OH)2) –?
с (NH3(p)) –?
Розв’язування
Виходячи з добутку розчинності Mg(OH)2 визначають концентрацію йонів Mg2+ і рівну їй концентрацію Mg(OH)2 у водному насиченому розчині.
ДР (Mg(OH)2) = [Mg2+]·[OH–]2 = 6,0∙10–10.
Позначимо через х моль/дм 3 розчинність Mg(OH)2. Тоді [Mg2+] = х моль/дм 3, а [OH–] = 2 х моль/дм 3. Підставляючи ці значення у формулу добутку розчинності Mg(OH)2, маємо:
х ·(2 х)2 = 6,0·10–10; 4 х 3 = 6,0·10–10; х 3 =  ;
;
х =  = 5,31·10–4 моль/дм 3.
= 5,31·10–4 моль/дм 3.
Отже, втрати при промиванні осаду Mg(OH)2 водою за рахунок розчинності будуть складати:
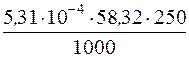 = 7,74·10–3 г Mg(OH)2,
= 7,74·10–3 г Mg(OH)2,
або  = 3,87%.
= 3,87%.
Якщо промивати осад Mg(OH)2 розчином амоніаку, розчинність магній гідроксиду знижується внаслідок введення однойменних з осадом йонів ОН–. Знаючи, що втрати Mg(OH)2 при промиванні цією промивною рідиною не повинні перевищувати 0,1%, знаходимо розчинність Mg(OH)2 в моль/дм 3:
 = 1,37·10–5 моль/дм 3.
= 1,37·10–5 моль/дм 3.
Отже, [Mg2+] = 1,37·10–5 моль/дм 3.
Використовуючи значення добутку розчинності Mg(OH)2, визначаємо рівноважну концентрацію ОН–-іонів:
6,0·10–10 = [Mg2+]·[OH–]2 = 1,37·10–5·[OH–]2
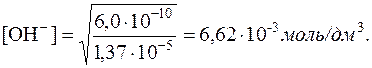
На основі константи дисоціації NH3·H2O

розраховуємо концентрацію NH3 у розчині, у якому [OH–] =
6,62·10–3 моль/дм 3. Так як у водному розчині амоніаку [  ]=[OH–], то з константи дисоціації виходить:
]=[OH–], то з константи дисоціації виходить:

Приклад 6. Після відповідної обробки мінералу, що містить Сульфур, взятого масою 2,6248 г було отримано 0,3248 г BaSO4. Розрахувати масову частку Сульфуру у досліджуваному мінералі.
Дано:
m (мінералу) = 2,6248г M (S) = 32,06 г/моль
m (BaSO4) = 0,3248г M (BaSO4) = 233,39 г/моль
 w (S) –?
w (S) –?
Розв’язування
Фактор перерахунку (гравіметричний множник):
 .
.
Тоді:

Масова частка Сульфуру у досліджуваному мінералі складає 1,7%.
§ 32. Запитання для самоперевірки
1. Які вимоги ставляться до осаджуваної форми у гравіметричному аналізі?
2. Які вимоги ставляться до гравіметричної форми?
3. Як впливають на повноту осадження BaSO4 температура розчину, концентрація і об’єм розчину осаджувача, наявність сторонніх електролітів?
4. Які йони будуть адсорбуватися на поверхні осаду на початку осадження BaCl2 розчином Na2SO4 і Na2SO4 розчином BaCl2?
5. Які йони будуть адсорбуватися на поверхні осаду при додаванні надлишку осаджувача після осадження BaCl2 розчином Na2SO4 і Na2SO4 розчином BaCl2?
6. Яку сіль – Ba(NО3)2, BaBr2, BaCl2 чи Ba(ClO4)2 – доцільно використати як осаджувач для отримання найбільш чистого осаду BaSO4?
7. Яку сполуку – K2C2O4, Na2C2O4, H2C2O4 чи (NH4)2C2O4 – доцільно використати як осаджувач для осадження йонів Ca2+?
8. У якому випадку втрати при промиванні осаду Fe(OH)3 будуть найменшими: а) при промиванні осаду водою; б) розчином NH4NO3; в) розчином NH4NO3 з NH3?
9. Яка сполука найбільш придатна як гравіметрична форма для кількісного визначення Ca2+, Sr2+, Ni2+, Zn2+, Cu2+?
10. Як зв’язано відносне пересичення розчину з числом центрів кристалізації, швидкістю кристалізації, розміром кристалів?
11. Які процеси відбуваються при дозріванні кристалічних осадів?
12. Чому осадження барій сульфату проводиться з розведених розчинів, у кислому середовищі, при нагріванні розчинів, у присутності солей амонію?
13. Які фізико-хімічні процеси у розчині призводять до співосадження?
14. Які аналітичні заходи застосовуються для зменшення адсорбції і оклюзії при отриманні кристалічних осадів?
15. Які умови осадження аморфних осадів? Чому їх осадження проводять з концентрованих розчинів?






