Окислительно-восстановительными называются химические реакции, сопровождающиеся изменением степени окисления атомов элементов.
Окислением называется процесс отдачи электронов, а восстановлением процесс принятия электронов. Окисление и восстановление взаимосвязаны.
Окислителем называется вещество, атомы которого принимают электроны, при этом он восстанавливается. Восстановителем называется вещество, атомы которого отдают электроны, при этом он окисляется.
Все окислительно-восстановительные реакции классифицируют следующим образом:
1. Межмолекулярные реакции. Это реакции, в которых окислитель и восстановитель являются различными веществами.
 ,
,
где Mn+4 – окислитель, Cl–1 – восстановитель.
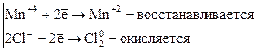 .
.
2. Реакции внутримолекулярного окисления. Это реакции, которые протекают с изменением степеней окисления атомов различных элементов одного и того же вещества.
 ,
,
где Mn+7 – окислитель, а O-2 – восстановитель.

3. Реакции диспропорционирования. В этих реакциях и окислителем и восстановителем является элемент находящийся в промежуточной степени окисления в составе одного и того же вещества.
 ,
,
где Cl20 – окислитель и восстановитель.

О возможности того или иного вещества проявлять окислительные, восстановительные или двойственные свойства можно судить по степени окисления элементов, выполняющих эти функции.
Элементы в своей высшей степени окисления проявляют только окислительные свойства, а в низшей степени окисления проявляют только восстановительные свойства. Элементы, имеющие промежуточную степень окисления, могут проявлять как окислительные, так и восстановительные свойства. Некоторые окислители и восстановители приведены ниже.
| Окислители | Схемы реакций | |||
| KMnO4 |    
| |||

|   
| |||
| H2O2 | 
| |||
| Восстановители | Схемы реакций | |||
| H2S и ее соли, Na2S2O3 | 
| |||
| НГ и их соли |  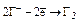
| |||
| Cr+3 |  
| |||
| HNO2 и ее соли. |  (HNO3 или ее соли). (HNO3 или ее соли).
| |||
| H2SO3 и ее соли. |  (H2SO4 или ее соли). (H2SO4 или ее соли).
| |||
| H2O2 | 
| |||
Примеры решения задач
Пример 1. составить электронную схему и закончить уравнение реакции: FeSO4 + K2Cr2O7 + H2SO4 → …
Решение. Степени окисления изменяются у железа Fe+2 и хрома Cr+6. Составим уравнение баланса электронов, причем расчет ведем на два атома хрома (по числу атомов в молекуле K2Cr2O7):
 6 Fe+2 – ē → Fe+3
6 Fe+2 – ē → Fe+3
 1 2Cr+6 + 6ē→ 2Cr+3
1 2Cr+6 + 6ē→ 2Cr+3
6 Fe2+ + 2 Cr+6 → 2 Cr+3 + 6 Fe+3
Расставим полученные коэффициенты в левую и правую части исходного уравнения. Учитывая, что реакция протекает при избытке серной кислоты, конечным продуктом реакции будут сульфаты всех металлов. Водород образует воду. Недостаток сульфат-ионов слева компенсируют семью молями серной кислоты. В последнюю очередь уравнивают реакцию по водороду (7 Н2О). Проверку следует вести по кислороду (по 59 атомов слева и справа).
Окончательно
6 FeSO4 + K2Cr2O7 + 7 H2SO4 →
→ 3 Fe2(SO4)3 +Cr2(SO4)3 + K2SO4 + 7 H2O
Пример 2. Закончить и уравнять реакцию:
С6Н12О6 + КМnО4 + Н2SО4 → СО2 + …
Решение. Необходимо составить схему процесса. В кислой среде перманганат- ион восстанавливается до иона марганца со степенью окисления +2. Для уравнивания числа атомов кислорода добавляют воду или ионы Н+ в кислой среде или ОН- в щелочной:
МnО4- + 8 Н+ + 5 e- → Мn2+ + 4 Н2О
Восстановитель С6Н12О6 окисляется до СО2, согласно полуреакции:
С6Н12О6 + 6 Н2О - 24 e- → 6 СО2 + 24 Н+
Приводим число электронов к наименьшему общему кратному, в примере к 120. Для этого уравнения полуреакций домножают на соответствующие коэффициенты, в примере на 24 и 5. Суммируют уравнения полуреакций, сокращая одинаковые члены в левой и правой частях. В результате получают ионное уравнение реакции:
5 С6Н12О6 + 24 МnО4- + 72 Н+ → 30 СО2 + 24 Мn2+ + 66 Н2О
Составляют молекулярное уравнение реакции путем добавления к ионам имеющихся в растворе противоионов:
5 С6Н12О6+24 КМnО4+36 Н2SO4 = 30 СО2+24 МnSO4+66 Н2О+12 К2SО4
Задачи для решения
I. Дописать схемы окислительно-восстановительных реакций и расставить коэффициенты в уравнениях.
1. MnO2+KClO3+KOH → K2MnO4+KCl+…
2. HgS+HNO3+HCl → S+NO+…
3. SO2+Br2+H2O → H2SO4+…
4. Mn(OH)2+Cl2+KOH → MnO2+KCl+…
5. K[Cr(OH)4]+Br2+KOH → K2CrO4+KB2+…
6. Cl2+S+H2O → H2SO4+…
7. K2MnO4+Cl2 → KMnO4+…
8. KNO3 → KNO2+O2+…
9. AgNO3 → Ag+NO2+O2+…
10. Pb(NO3)2 → PbO+NO2+O2+…
11. NO2+H2O → HNO3+NO+…
12. Ca(OH)2+NO2 → Ca(NO3)2+ Ca(NO2)2+…
13. KClO3 → KClO4+KCl+…
14. I2+ Ba(OH)2 → Ba(IO3) 2+ BaI2+…
15. H2S+O2 → SO2+…
16. S+HNO3 → H2SO4+ NO2+…
17. C+HNO3(K) → CO2+ NO+…
18. NH3+O2 → NO+…
19. H2O2 +KMnO4+ H2SO4 → O2+…
20. K3[Cr(OH)6]+Br2+KOH → K2CrO4+…
21. H2S+K2Cr2O7+H2SO4 → S+…
22. KNO2+KMnO4+H2O → K3NO3+…
23. KNO2+KMnO4+KOH → KNO3+…
24. K4[Fe(CN)6]+H2O2+H2SO4 → K3[Fe(CN)6]+…
25. K3[Fe(CN)6]+H2O2+KOH →K3[Fe(CN)6]+O2+…
26. Zn+KClO3+KOH → K2[Zn(OH)4]+KCl+…
27. Al+KNO3+KOH → K3[Al(OH)6]+NH3+…
28. Al+NaOH+H2O → Na3[Al(OH)6]+…
29. K2[Sn(OH)4]+KOH+Bi(NO3)3 → K2[Sn(OH)6]+Bi+…
30. SnCl2+HCl+HNO3 → H2[SnCl6]+NO+…
31. SnCl2+HgCl2+HCl → Hg+H2[SnCl6]+…
32. KNO2+KMnO4+H2SO4 → KNO3+…
33. Cu2O+HNO3 → NO2+…
34. FeSO4+KClO3+H2SO4 → KCl+…
35. CuCl2+SO2+H2O → CuCl+…
36. CrCl3+Br2+KOH → K2CrO4+…
37. H2O2+KI+H2SO4 → I2+…
38. H2S+ H2O2 → H2SO4+…
39. H2S+H2SO3 →…
40. H2O2+HIO3 → I2+…
41. H2O2+K2Cr2O7+H2SO4 →…
42. K2Cr2O7+H2S+H2SO4 → SO2+…
43. Hg2(NO3)2+O2+HNO3 → Hg2(NO3)2+…
44. NaCrO2+H2O2+NaOH → Na2CrO4+…
45. HBr+HBrO3 → Br2+…
46. HClO+H2O2 → HCl+…
47. CrO3+HCl → Cl2+…
48. PH3+KMnO4+H2SO4 → H3PO4+…
49. NaBr+NaBrO3+H2SO4 → Br2+…
50. SnS+HNO3 → NO+…
51. HgI2+H2O2+H2SO4 → I2+…
52. KMnO4+MnSO4+H2O → MnO2+…
53. H2MnO4 → HMnO4+MnO2+…
54. Hg+HNO3(в.р.) → NH3+…
55. K2Cr2O7+SnCl2+HCl → K2[SnCl6]+…
56. Na[Cr(OH)4]+NaClO3+NaOH → Na2CrO4+NaCl+…
57. FeS2+HNO3 → Fe(NO3)3+NO+…
58. Fe3O4+HNO3 → Fe2(SO4)3+SO2+…
59. Fe3O4+H2SO4 → Fe2(SO4)3+SO2+…
60. CuFeS2+HNO3 → FeSO4+CuSO4+NO+…
61. As2S2+HNO3 → H3AsO4+H2SO4+NO+…
62. Zn+HNO3(в.р.) → N2+Zn(NO3)2+…
63. SnCl2+Cl+KOH →…
64. MnSO4+Br2+H2O → HMnO4+…
65. FeSO4+KBrO3+H2SO4(pазб) →…
66. Fe(OH)3+Cl2+KOH → KClO3+…
67. HClO+KI →…
68. HClO+H2S →…
69. HClO4+H2SO3 →…
70. HClO4+C →…
71. K2S+KMnO4+H2SO4(разб.) →…
72. P+KOH+H2O → KH2PO4+…
73. NO2+KOH → KNO3+…
74. K2MnO4+H2SO4(разб.) → MnO2+KMnO4+…
75. MnSO4+NaBiO3+H2SO4(разб.) → …
76. KMnO4+NaI+H2O →…
77. MnSO4+PbO2+HNO3 → HMnO4+…
78. MnSO4+KClO3+H2O → HMnO4+…
79. KMnO4+H2O2+H2O →…
80. SnCl2+NaBiO3+HClp → SnCl4+…
81. Cl2+I2+H2O →…
82. HBrO+HBr →…
83. Ca(ClO2)2+NaI+H2SO4 →…
84. NaClO+Na2S+H2SO4(разб.) →…
85. NaIO3+Cl2+KOH →…
86. KClO3+S →…
87. NaClO3+MnO2+NaOH → Na2MnO4+…
88. H2O2+NaI →…
89. H2O2+CrCl3+KOH → K2CrO4+…
90. H2O2+KMnO4 → MnO2+…
91. H2O2+K2Cr2O7+HClp →…
92. H2S+KMnO4+H2O →…
93. SO2+KMnO4+H2O →…
94. SO2+K2Cr2O7+H2SO4(разб.) →…
95. SO2+Br2+H2O →…
96. H2SO4(конц.)+Mg → H2S+…
97. H2O2+SO2 →…
98. H2S+SO2+NaOH →…
99. PH3+KMnO4+ H2SO4(разб.) → H3PO4+…
100. P+KOH →…
II. Написать уравнения окислительно-восстановительных реакций и расставить коэффициенты, пользуясь методом ионно-электронного баланса.
В сернокислой среде
101. Перманганатом калия и сульфитом калия
102. Перманганатом и нитритом калия
103. Перманганатом и гидросульфитом калия
104. Перманганатом калия и сульфитом железа (II)
105. Перманганатом калия и сульфитом олова (II)
106. Перманганатом калия и пероксидом водорода
107. Перманганатом калия и сульфидом калия
108. Перманганатом калия и сероводородом
109. Перманганатом калия и соляной кислотой (конц.)
110. Перманганатом калия и бромистоводородной кислотой
111. Перманганатом калия и бромистоводородной кислотой
112. Перманганатом калия и иодидом калия
113. перманганатом калия и бромидом калия
114. перманганатом кальция и сульфитом калия
115. перманганатом кальция и хлоридом олова (II)
116. Перманганатом кальция и нитритом калия
117. Дихроматом калия и сульфитом калия
118. Дихроматом калия и нитритом калия
119. дихроматом калия и сульфатом железа (II)
120. Дихроматом калия и сульфатом олова (II)
121. Дихроматом калия и пероксидом водорода
122. Дихроматом калия и сульфидом натрия
123. Дихроматом калия и диоксидом серы
124. Дихроматом калия и бромистоводородной кислотой
125. Дихроматом калия и иодистоводородной кислотой
126. Дихроматом калия и бромидом калия
127. Дихроматом калия и иодидом калия
128. Дихроматом калия и гидросульфитом калия
129. Хроматом калия и сульфитом калия
130. Хроматом калия и нитритом калия
131. Манганатом калия и сульфидом калия
132. Манганатом калия и иодидом калия
133. Манганатом калия и бромидом калия
134. Хлоратом калия и сульфатом железа (II)
135. Хлоратом калия и сульфатом олова (II)
В солянокислой среде
136. Переманганатом калия и сульфитом олова (II)
137. Переманганатом калия и хлоридом железа (II)
138. Переманганатом калия и хлоридом олова (II)
139. Дихроматом калия и хлоридом олова (II)
140. Дихроматом калия и соляной кислотой (конц.)
141. Хроматом калия и соляной кислотой (конц.)
142. Хроматом калия и хлоридом олова (II)
143. Манганатом калия и соляной кислотой (конц.)
144. Хроматом калия и соляной кислотой (конц.)
145. Броматом калия и хлоридом железа (II)
146. Броматом калия и хлоридом олова (II)
147. Иодатом калия и хлоридом олова (II)
148. Диоксидом марганца и хлоридом железа (II)
149. Диоксидом марганца и хлоридом олова (II)
В щелочной среде – NaOH KOH
150. Иодом и бромидом олова (II)
151. Пермаганатом калия и хлоридом олова (II)
152. Пермаганатом калия и сульфатом олова (II)
153. Гипохлоритом натрия и сульфатом олова (II)
154. Гипобромитом калия и хлоридом олова (II)
155. Гипоиодитом калия и сульфатом олова (II)
156. Пероксидом водорода и алюминием
157. Бромом и сульфатом олова (II)
158. Хлором и хлоридом олова (IV)
159. Фтором и хлоридом олова (IV)
160. Иодом и сульфитом натрия
161. Бромом и сульфитом натрия
162. Хлором и сульфитом натрия
163. Фтором и сульфитом калия
164. Иодом и серой
165. Пероксидом водорода и серой
166. Гипоиодидом калия и алюминием
167. Гипоиодитом бария и алюминием
168. Пероксидом водорода и алюминием
169. Хлором и серой
170. Метахромитом калия и бромом
171. Сурьмой и бромом
172. Арсином и иодом
173. Хлоратом натрия и оксидом марганца (IV)
174. Иодатом калия и хлором
175. Сероводородом и оксидом серы (IV)
176. Хлоридом олова (II) и хлором
177. Сульфатом хрома (II) и бромом
178. Метахромитом натрия и оксидом свинца (II)
179. Сульфатом марганца (II) и бромом
180. Метахромитом марганца (II) и пероксидом водорода
В нейтральной среде (H2O)
181. Оксидом серы (IV) и бромом
182. Серой и хлором
183. Сероводородом и хлором
184. Пермаганатом калия и сульфитом калия
185. Пермагнатом калия и нитритом кальция
186. Пермаганатом калия и оксидом азота (IV)
187. Оксидом серы (IV) и ортомышьяковой кислотой
188. Гидроксидом никеля (II) и гипохлоритом натрия
189. Хлорной кислотой и оксидом серы (IV)
190. Сульфитом железа (III) и оксидом серы (IV)
191. Сульфитом калия и бромом
192. Перманганатом калия и сульфатом марганца (II)
193. Метахромитом калия и пероксидом водорода
194. Гидроксидом железа (II) и кислородом
195. Алюминием и гидроксидом натрия
196. Пермаганатом калия и сульфатом хрома (III)
197. Пермаганатом калия и иодидом калия
198. Пермагнатом калия и пероксидом водорода
199. Сульфитом калия и сероводородом
200. Нитритом натрия и иодидом калия
Приложение 1
Константы диссоциации некоторых слабых кислот и оснований
| Название | Химическая формула | Kd |
| Кислоты | ||
| Азотистая | HNO2 | 6,9·10–4 |
| Бензойная | C6H5COOH | 6,3·10–5 |
| Борная (мета) | HBO2 | 7,5·10–10 |
| Борная (орто) | H3BO3 | 5,83·10–10 |
| 1,8·10–13 | ||
| 1,6·10–14 | ||
| Бромноватистая | HBrO | 2,2·10–9 |
| Винная | C4H6O6 | 1,3·10–3 |
| 3,0·10–5 | ||
| Германиевая | H2GeO3 | 1,7·10–9 |
| 1,9·10–13 | ||
| Кремниевая (мета) | H2SiO3 | 4,54·10–10 |
| 1,0·10–13 | ||
| Лимонная | C6H8O7 | 7,45·10–4 |
| 1,73·10–5 | ||
| 4,02·10–6 | ||
| Муравьиная | HCOOH | 1,77·10–4 |
| Ортофосфорная | H3PO4 | 7,11·10–3 |
| 6,34·10–8 | ||
| 1,26·10–12 | ||
| Плавиковая | HF | 6,2·10–4 |
| Селеноводородная | H2Se | 1,3·10–4 |
| 1,0·10–11 | ||
| Сернистая | H2SO3 | 1,4·10–2 |
| 6,2·10–8 | ||
| Сероводородная | H2S | 1,1·10–7 |
| 3,6·10–12 | ||
| Синильная | HCN | 5·10–10 |
| Угольная | H2CO3 | 4,45·10–7 |
| 4,69·10–11 | ||
| Уксусная | CH3COOH | 1,75·10–5 |
| Фенол | C6H5OH | 1,01·10–10 |
| Щавелевая | H2C2O4 | 6,5·10–2 |
| 5,18·10–5 | ||
| Основания | ||
| Анилин | C6H5NH2OH | 3,82·10–10 |
| Бензиламин | C7H8NH2OH | 2,35·10–5 |
| Бутиламин | C4H10NH2OH | 4,57·10–4 |
| Гидразин | N2H5OH | 1,7·10–6 |
| Гидроксид аммония | NH4OH | 1,77·10–5 |
| Гидроксиламин | NH2OH·H2O | 8,9·10–9 |
| Диметиламин | (CH3)2NH2OH | 6,0·10–4 |
| Диэтиламин | (C2H5)2NH2OH | 9,6·10–4 |
| Метиламин | CH4NH2OH | 4,24·10–4 |
| Пиридин | C5H12NH2OH | 1,71·10–9 |
| Пропиламин | C3H8NH2OH | 5,62·10–4 |
| Тиомочевина | CS(NH2)2·H2O | 1,1·10–12 |
| Триметиламин | (CH3)3NH2OH | 6,31·10–5 |
| Хинолин | C9H6NH2OH | 1,0·10–9 |
| Этаноламин | C2H6ONH2OH | 3,0·10–5 |
| Этиламин | C2H6NH2OH | 3,18·10–4 |
Приложение 2
Константы нестойкости гидроксокомплексов
| Ион | p Kn = -lg Kn |
| CdOH+ | 2,4 |
| CoOH+ | 4,4 |
| CrOH+ | 7,8 |
| CuOH+ | 6,0 |
| NiOH+ | 4,6 |
| PbOH+ | 6,22 |
| ZnOH+ | 5,7 |
Приложение 3
Произведения растворимости
| Вещество | L | Вещество | L | |
| Ag2C2O4 | 3,5·10–11 | Fe(OH)3 | 6,3×10-38 | |
| Ag2CO3 | 1,2×10-12 | Hg2Br2 | 5,4×10-23 | |
| Ag2CrO4 | 4,7×10-12 | Hg2Cl2 | 1,2×10-18 | |
| Ag2S | 4,23×10-50 | Hg2CrO4 | 5,0×10-9 | |
| Ag2SO4 | 1,24×10-5 | Hg2I2 | 4,4×10-29 | |
| Ag3AsO4 | 1·10–22 | Hg2SO4 | 6,4×10-7 | |
| Ag3PO4 | 1,3×10-20 | In4[Fe(CN)6]3 | 1,9×10-44 | |
| AgBr | 4,8×10-13 | K2SiF6 | 8,7×10-7 | |
| AgBrO3 | 6,1×10-5 | La(OH)3 | 6,5×10-20 | |
| AgCl | 1,73×10-10 | LaF3 | 5×10-14 | |
| AgI | 8,1×10-17 | Li3PO4 | 3,2×10-9 | |
| AgIO3 | 3,0×10-8 | Mg(OH)2 | 6,0×10-10 | |
| Al(OH)3 | 1×10-32 | Mn(OH)2 | 1,9×10-13 | |
| Ba3(AsO4)2 | 7,8×10-51 | Na3AlF6 | 4,1×10-10 | |
| Ba3(PO4)2 | 6,0×10-39 | NaBeF6 | 7,0×10-3 | |
| BaCrO4 | 1,2×10-10 | Ni(OH)2 | 2,0×10-15 | |
| BaSO3 | 8,0×10-7 | Pb(OH)2 | 5×10-16 | |
| BaSO4 | 1,0 ×10-10 | Pb3(PO4)2 | 7,9×10-43 | |
| Be(OH)2 | 6,3×10-22 | PbBr2 | 4,5×10-6 | |
| Bi(OH)3 | 3×10-32 | PbCl2 | 1,6×10-5 | |
| Bi2(C2O4)3 | 4,0×10-36 | PbCO3 | 7,5×10-14 | |
| Ca(OH)2 | 5,5×10-6 | PbCrO4 | 1,8·10–14 | |
| Ca3(PO4)2 | 2,0×10-29 | PbI2 | 8,2×10-9 | |
| CaC2O4 | 2,3·10–9 | PbS | 6,2×10-28 | |
| CaCO3 | 3,7×10-9 | PbSO4 | 1,3×10-8 | |
| CaF2 | 4,0×10-11 | PtCl4 | 8,0×10-29 | |
| CaHPO4 | 1,4×10-6 | PuO2(OH)2 | 3,2×10-21 | |
| CaSO4 | 1,7×10-5 | Sb(OH)3 | 4×10-42 | |
| Cd(OH)2 | 2,2×10-14 | Sc(OH)3 | 2×10-30 | |
| CdCO3 | 2,5×10-14 | Sn(OH)2 | 6,3×10-27 | |
| Co(OH)2 | 6,3×10-15 | Sn(OH)4 | 1×10-57 | |
| Cr(OH)3 | 6,3×10-31 | Sr(OH)2 | 3,2×10-4 | |
| Cu(OH)2 | 2,2×10-20 | SrCrO4 | 3,6·10–5 | |
| CuCl | 3,2×10-7 | SrSO4 | 3,2·10–7 | |
| CuI | 1,1×10-12 | Tl(OH)3 | 6,3×10-46 | |
| Продолжение приложения 3 | ||||
| Вещество | L | Вещество | L | |
| Fe(OH)2 | 8×10-16 | TlBr | 3,6×10-6 | |
| Y(OH)3 | 3,2×10-25 | TlCl | 1,8×10-4 | |
| TlI | 8,8×10-8 | SnS | 1·10–25 | |
| Tl2S | 5,0·10–21 | Zn(OH)2 | 1,2·10–17 | |
| Zn3(AsO4)2 | 1,3·10–27 | ZnS | 1,9·10–22 | |
| Zr(OH)4 | 1,0·10–52 |
Оглавление
1. Основные классы неорганических соединений. Номенклатура. 3
1.1. Оксиды.. 3
1.2. Гидроксиды.. 4
1.3. Кислоты.. 5
1.4. Соли. 7
1.5. Задачи для решения. 9
2. Строение атома. 15
2.1. Примеры решения задач. 17
2.2. Задачи для решения. 19
4. Основные понятия и законы химии. 21
4.1. Моль, молярная масса. 21
4.2. Основные газовые законы.. 22
4.3. Закон эквивалентов. 23
4.4. Примеры решения задач. 24
4.5. Задачи для решения. 27
5. Растворы.. 44
5.1. Концентрации растворов. 44
5.1.1. Примеры решения задач. 45
5.1.2. Задачи для решения. 48
5.2. Коллигативные свойства растворов. 58
5.2.1. закон Рауля. 58
5.2.2. Изменение температур фазовых переходов растворов. 59
5.2.3. Осмотическое давление. 59
5.2.4. Примеры решения задач. 60
5.2.5. Задачи для решения. 62
5.3. Водородный показатель. 68
5.3.1. Расчет рН в растворах сильных кислот и оснований. 69
5.3.2. Расчет рН в растворах слабых кислот и оснований. 70
5.3.3. Примеры решения задач. 71
5.3.4. Задачи для решения. 74
5.4. Гидролиз. 83
5.4.1. Примеры решения задач. 86
5.4.2. Задачи для решения. 88
5.5. Равновесия в буферных растворах. 90
5.5.1. Примеры решения задач. 93
5.5.2. Задачи для решения. 95
5.6. Равновесия в насыщенных растворах. 98
5.6.1. Растворимость в бинарной системе соль - вода. 99
5.6.2. Растворимость в многокомпонентной системе с одноименными ионами. 99
5.6.3. Условия образования осадков. 100
5.6.4. Примеры решения задач. 100
5.6.5. Задачи для решения. 102
6. Окислительно-восстановительные реакции. 106
6.1. Примеры решения задач. 108
6.2. Задачи для решения. 109
Приложение 1. 116
Приложение 2. 118
Приложение 3. 119






