333. Ca(NO3)2 + Na3PO4®.
334. Ca(OH)2 + CO2 ®.
335. CaCl2 + H2SO4 ®.
336. CaCO3 + CH3COOH ®.
337. CaCO3 + HCl ®.
338. CaCO3 + CO2 + H2O ®.
339. Cd(NO3)2 + Na2S ®.
340. CdCl2 + H2S ®.
341. CH3COOAg + H2S®.
342. CH3COOH + Ba(OH)2 ®.
343. CH3COOH + NH4OH ®.
344. CH3COOK + H2SO4 ®.
345. HCOOK + H2SO4 ®.
346. CH3COOPb + Na2CrO4 ®.
347. CH3COOPb + Na2SO4 ®.
348. Cr2(SO4)3 + NaOH ®.
349. Cr2(SO4)3 + NH4OH ®.
350. Cu(NO3)2 + Ba(OH)2 ®.
351. Cu(NO3)2 + H2S ®.
352. Cu(NO3)2 + Na2S ®.
353. CuSO4 + NaOH ®.
354. CuSO4 + NH4OH ®.
355. CuSO4 + Ba(OH)2 ®.
356. Fe(OH)3 + H2SO4 ®.
357. Fe2(SO4)3 + NaOH ®.
358. FeCl3 + KOH ®.
359. FeOHCl2 + HCl ®.
360. FeS + HCl ®.
361. H2S + NH4OH ®.
362. HCOOK + HNO3 ®.
363. HF + KOH ®.
364. Hg(NO3)2 + H2S ®.
365. HNO2 + NH4OH ®.
366. K2CO3 + H3PO4 ®.
367. K2CO3 + HCl ®.
368. KHSO3 + H2SO4 ®.
369. KOH + HCN ®.
370. KOH + H3PO4 ®.
371. Mg(NO3)2 + (NH4)2C2O4 ®.
372. MgCO3 + HCl ®.
373. MgOHCl + HCl ®.
374. MnCl2 + NH4OH ®.
375. Na2S + H2SO4 ®.
376. Na2S + NiSO4 ®.
377. Na2S + CdSO4®.
378. Na2SiO3 + H2SO4 ®.
379. Na2SO4 + BaCl2 ®;
380. Na2SO4 + H2SO4 ®.
381. Na2SO4 + Pb(NO3)2 ®.
382. Na2SO4 + BaCl2®.
383. NaHCO3 + HCl ®.
384. NaHCO3 + NaOH ®.
385. NaHPO4+NaOH ®.
386. NaHSO4 + NaOH ®.
387. NaHSO4 + Ba(NO3)2 ®.
388. NaOH + H2SO3 ®.
389. NaOH + H2SO4 ®.
390. NH4Cl + Ca(OH)2 ®.
391. NH4Cl + NaOH ®.
392. NH4Cl + Sr(OH)2 ®.
393. NH4Cl + Ba(OH)2 ®.
394. Ni(NO3)2 + KOH ®.
395. NiSO4 + (NH4)2S ®.
396. Pb(NO3)2 + Fe2(SO4)3 ®.
397. Pb(NO3)2 + K2CrO4 ®.
398. Pb(NO3)2 + KI ®.
399. Pb(NO3)2 + Na2S ®.
400. Pb(NO3)2 +NaCl ®.
401. Sr(NO3)2 + H2SO4 ®.
402. Zn(NO3)2 + KOH ®.
403. Zn(OH)2 + H2SO4 ®.
404. Zn(OH)2 + NaOH(избыток) ®.
405. Zn(OH)2+NaOH ®.
2. Строение атома
Атом любого элемента состоит из положительно заряженного ядра и отрицательно заряженных электронов, в целом же атом – система электронейтральная. Заряд ядра равен порядковому номеру элемента в таблице Д.И. Менделеева. Состояние электрона в атоме описывается при помощи набора четырех квантовых чисел: главного n, орбитального l, магнитного ml и спинового ms. Определенные значения трех квантовых чисел (n, l,ml) описывают состояние электрона называемое атомной орбиталью (АО).
Главное квантовое число n определяет энергию АО и номер энергетического уровня, на котором находится электрон, может принимать целочисленные значения от единицы до бесконечности.
Орбитальное квантовое число l определяет форму АО и энергетический подуровень, может принимать значения от нуля до n -1. Исторически атомным орбиталям со значениями l, равным 0, 1, 2, 3 присвоены буквенные обозначения s -, p -, d -, f -. В графических схемах электронного строения атомов каждая орбиталь обозначается символом ра
Магнитное квантовое число ml определяет пространственную ориентацию данной АО и отчасти ее форму, может принимать значения от – l …0…+ l.
Спиновое квантовое число m s характеризует собственный момент импульса и связанный с ним магнитный момент, может принимать значения ±1/2.
Последовательность распределения электронов в атоме по мере увеличения значений l и n выражается электронными или электронно-графическими формулами.
При заполнении АО действует принцип Паули, из которого следует, что в атоме не может быть двух электронов, характеризующихся одинаковым набором значений четырех квантовых чисел. Состояние электронов в атоме должно отличаться значением хотя бы одного квантового числа.
Заполнение энергетических подуровней подчиняется правилу Хунда, согласно которому электроны в основном состоянии в атоме располагаются так, чтобы модуль суммарного спина всех электронов подуровня был максимальным. Например, четыре валентных p -электрона атома кислорода размещаются в квантовых ячейках следующим образом:

Последовательность заполнения энергетических уровней и подуровней в атомах выражается правилом Клечковкого: порядок заполнения определяется возрастанием суммы n + l, а при одинаковом ее значении первым заполняется подуровень с меньшим значением n в этой сумме:
1 s ®2 s ®2 p ®3 s ®3 p ®4 s ®3 d ®4 p ®5 s ®4 d ®5 p ®6 s ®5 d 1(La)®4 f (лантаноиды)®5 d ®6 p ®7 s ®6 d 1(Ac)®5 f (актиноиды)®6 d ®…
Принадлежность элемента к электронному семейству определяется характером заполнения энергетических подуровней: s -элементы – заполнение внешнего s -подуровня, например: Li: 1 s 2 2 s 2; р -элементы – заполнение внешнего p -подуровня, например: F: 1 s 22 s 2 2 p 5; d -элементы – заполнение предвнешнего d -подуровня, например: V: 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 3; f -элементы – заполнение f -подуровня второго снаружи уровня, например:  Nd: 1 s 2 2 s 2 2 p 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 10 4 p 6 5 s 2 4 d 10 5 p 6 6 s 2 4 f 4.
Nd: 1 s 2 2 s 2 2 p 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 10 4 p 6 5 s 2 4 d 10 5 p 6 6 s 2 4 f 4.
Для d - и f -элементов имеются отклонения от описанного способа заполнения АО – так называемый провал электрона. Это явление связано с тем, что для атома устойчивым состоянием является полностью или на половину заполненная АО, т. е. d 10, d 5, f 14, f 7. В ситуации, когда до достижения такого состояния не хватает одного электрона, он переходит («проваливается») с предыдущего уровня, например электронный паспорт серебра 1 s 22 s 22 p 63 s 23 p 64 s 23 d 104 p 6 5 s 24 d 9 с учетом провала электрона: 1 s 22 s 22 p 63 s 23 p 64 s 23 d 104 p 6 5 s 14 d 10.
Если на валентных энергетических уровнях имеются вакантные АО, то при получении электронами порции энергии (возбуждении атома) становится возможным «разъединение» валентных электронов, то есть их переходы с тех подуровней, где все АО заняты полностью (¯) или частично () на другие валентные подуровни того же уровня, имеющие незаполненные АО. При этом с тех АО, которые в основном (соответствующем минимальной энергии атома) состоянии были заняты полностью, «уходит» по одному электрону последовательно, т. е. возможно несколько возбужденных состояний. Возбуждение меняет валентное состояние атома (число его неспаренных электронов).
Примеры решения задач
Пример 1. Составить электронную формулу атома брома и графическую схему заполнения электронами валентных орбиталей в нормальном и возбужденном состояниях.
Решение. 1. Порядковый номер брома – 35, следовательно атом серы имеет 35 электронов. Бром находится в четвертом периоде периодической системы, следовательно, АО с n = 1, 2 и 3 заполнены полностью. Бром относится к p -элементам, следовательно, заполнен 4 s -подуровень. В ряду 4 p -элементов бром – пятый элемент, следовательно, на 4 p -подуровне – 5 электронов. Таким образом, электронная формула брома - 1 s 22 s 22 p 63 s 23 p 44 s 23 d 104 p 5.
2. Валентными орбиталями в этом атоме являются орбитали внешнего (четвертого) электронного слоя, т. е. 4 s -, 4 p - и незаполненные 4 d -орбитали. Графически схема заполнения электронами этих орбиталей имеет следующий вид:

в таком состоянии бром имеет валентность 1, которой соответствуют степени окисления -1, +1.
3. При затрате некоторой энергии спаренный p -электрон перейдет на свободную d -орбиталь – первое возбужденное состояние брома:
Br*: 
в таком состоянии бром имеет валентность 3, которой соответствует степень окисления +3.
4. При передаче атому брома еще некоторого количества энергии следующий p -электрон также перейдет на свободную d -орбиталь – второе возбужденное состояние брома:
Br**: 
в таком состоянии бром имеет валентность 5, которой соответствует степень окисления +5.
5. При передаче атому брома еще некоторого количества энергии s -электрон также перейдет на свободную d -орбиталь – третье возбужденное состояние брома:
Br**: 
в таком состоянии бром имеет валентность 7, которой соответствует степень окисления +7.
Пример 2. Составить электронные формулы атома селена в состояниях Se-2 и Se+4 и графические схемы заполнения электронами валентных орбиталей.
Решение. 1. Cоставим электронную формулу атома селена (см. пример 1) 1 s 22 s 22 p 63 s 23 p 64 s 23 d 104 p 4 и графическую схему заполнения электронами валентных орбиталей

2. Для получения Se-2 необходимо к атому селена добавить два электрона на 4 p -орбиталь (согласно правилу Клечковского):

Электронная формула Se-2: 1 s 22 s 22 p 63 s 23 p 64 s 23 d 104 p 6.
3. Для получения Se+4 необходимо от атома селена убрать четыре электрона с 4 p -орбитали:

Электронная формула Se+4: 1 s 22 s 22 p 63 s 23 p 64 s 23 d 104 p 0.
Пример 3. Составить полную электронную формулу элемента, валентные электроны которого имеют конфигурацию 3 d 6, определить, к какому периоду таблицы Д. И. Менделеева принадлежит данный элемент.
Решение. Согласно правилу Клечковского 3 d -элементы находятся в четвертом периоде таблицы Д.И. Менделеева. На данной орбитали находится 6 электронов, значит это шестой по счету среди 3 d -элементов, т. е. железо. Полная электронная формула которого 1 s 22 s 22 p 63 s 23 p 64 s 23 d 6.
Задачи для решения
I. Составить электронные формулы элементов, графические схемы заполнения электронами валентных орбиталей в спокойном и возбужденном состояниях, указать, к какому типу эти элементы относятся.
1. B, Al, Th.
2. Po, Ba, Lu.
3. Mg, Pm, Be.
4. Br, Co, Hf.
5. C, Tm, As.
6. Nd, Ca, V.
7. Ta, O, Ce.
8. Y, Rb, S.
9. At, Xe, Lr.
10. Cs, U, H.
11. Cl, Cu, Bi.
12. Na, Ac, Fe.
13. Pb, Ra, Dy.
14. Ag, Re, In.
15. Cd, K, Pa.
16. P, Se, Li.
17. Yb, Mn, Sn.
18. Mo, La, N.
19. Pu, Ni, Sb.
20. Au, Np, Rn.
21. Cr, Tl, Cm.
22. Si, I, Zr.
23. Tb, Sr, Bk.
24. Fr, Ti, W.
25. He, Hg, Gd.
26. Pt, Ne, Sm.
27. Ga, Ru, Ho.
28. Sc, Pr, Os.
29. Ar, Ir, Eu.
30. Zn, Rh, Er.
31. Kr, Pd, Am.
32. Ge, Cf, F.
II. Составить электронные формулы атомов в указанных состояниях и графические схемы заполнения электронами валентных орбиталей.
33. Li+, C+2.
34. O-2, F-.
35. Na+, N-3.
36. Ca+2, C+4.
37. Al+3, B-3.
38. C-4, Ba+2.
39. S+6, P-3.
40. P+5, Cl-1.
41. I+5, Fe+3.
42. Be+2, Co+3.
43. Cr+6, Cu+2-.
44. I-, P+3.
45. Cr+3, Br+3.
46. Ag+, Sn+4.
47. Zn+2, S-2.
48. K+, Fe+2.
49. Zr+4, Pb+2.
50. N+5, Br-.
51. Ni+2, Cl+5.
52. Se-2, Cs+.
53. Te-2, Sr+2.
54. Bi+3, Si-4.
55. B+3, Sc+2.
56. Mg+2, Mn+7.
57. Cd+2, Sn+2-.
58. Nb+3, Hg+.
59. Tl+, V+3.
60. Ti+4, Mn+2.
61. Os+3, Au+3.
62. Rb+, Ce+3.
63. Fr+1, Y+3.
64. H+, Re+7.
III. Исходя из состояния валентных электронов, составить электронную формулу элемента в нулевой степени окисления. Определить, к какому периоду таблицы Д. И. Менделеева принадлежит данный элемент.
65. 4 d 1.
66. 3 d 10.
67. 4 s 13 d 10.
68. 5 d 2.
69. 6 p 2.
70. 6 s 14 f 145 d 10.
71. 4 s 23 d 5.
72. 4 s 13 d 5.
73. 7 s 26 d 1.
74. 5 d 3.
75. 6s24 f 2.
76. 6 p 1.
77. 5 s 14 d 5.
78. 4 f 3.
79. 6 d 15 f 3.
80. 5 s 24 d 5.
81. 5 d 8.
82. 5 s 14 d 10.
83. 5 s 24 d 10.
84. 5 s 2.
85. 5 p 3.
86. 6 p 4.
87. 4 d 6.
88. 5 d 6.
89. 5 f 5.
90. 5 d 7.
91. 5 f 2.
92. 4 d 7.
93. 5 d 14 f 7.
94. 4 f 10.
95. 4 d 8.
96. 5 p 6.
4. Основные понятия и законы химии
Моль, молярная масса
Известно, что любое вещество состоит из атомов, химические процессы протекают благодаря взаимодействию атомов. Из практических соображений было введено понятие моля. Условились считать, что 1 моль вещества содержит 6,02×1023 частиц. Любых – атомов, молекул, ионов. Значение 6,02×1023 называется числом Авогадро. Математически понятие моля можно записать в виде формулы:
 , ,
| (21) |
где n – количество вещества, моль, N – число частиц (молекул, атомов, ионов), N A – число Авогадро.
Массу 1 моль вещества называют молярной массой M (г/моль). Молярная масса в неорганической химии является характеристикой вещества, непосредственно связанной с его количественным составом и численно равна молекулярной массе (массе 1 молекулы) вещества, выраженной в углеродных единицах. Молярную массу любого вещества можно вычислить по формуле:
 , ,
| (22) |
где n i – стехиометрической индекс в формуле вещества, Мi – молярная масса элемента, входящего в соединение, г/моль – см. таблицу элементов Д.И. Менделеева.
Связь массы и количества вещества определяется формулой
 . .
| (23) |
Молярная масса вещества может быть определена экспериментально. Для газов ее находят, например, по относительной плотности газа D, которая представляет собой соотношение молярных масс двух газов, один из которых обычно известен:
 . .
| (24) |
Наиболее часто используют плотность газа по воздуху D возд., тогда М 2 = М возд. D возд. (М возд.= 29 г/моль), или водороду  , тогда М 2 =
, тогда М 2 =  .
.
Основные газовые законы
Состояние газа характеризуется его температурой, давлением и объемом. Если температура газа равна 0°С (273,15 K), а давление равно 1 атм. (1,013×105 Па = 760 мм. рт. ст.), то условия, при которых находится газ, называют нормальными.
Между объемом газа и его количеством вещества существует взаимосвязь, которая описывается законом Авогадро: в равных объемах любых газов, взятых при одной и той же температуре и одинаковом давлении, содержится одинаковое число молекул. Следовательно, при одинаковых условиях 1 моль любого газа занимает один и тот же объем. Этот объем называется молярным объемом газа VM. При нормальных условиях VM = 22,4 л и число моль газа может быть вычислено по уравнению
 . .
| (25) |
Взаимосвязь между количеством вещества газа, его температурой, давлением и объемом устанавливает уравнение Менделеева-Клайперона:
 , ,
| (26) |
где P – давление, Па; V – объем, м3; n – количество вещества, моль, m – масса, г; М – молярная масса газа, г/моль; R – универсальная газовая постоянная, в системе СИ R = 8,314 Дж/моль×K.
На практике чаще всего приходится иметь дело со смесями газов. Каждый газ вносит свой вклад в общее давление системы – парциальное давление. Парциальным давлением называется давление, которое производил бы этот газ, занимая при тех же физических условиях объем всей газовой смеси. Парциальное давление может быть вычислено через объемное содержание газа в газовой смеси:
 , ,
| (27) |
где Vi – объем данного газа, S Vi – общий объем газовой смеси,
или через мольную долю газа:
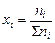 , ,
| (28) |
где ni – количество вещества данного газа, S ni – сумма числа моль всех компонентов газовой смеси
по уравнению:
| pi = xiP = j iP, | (29) |
где P – общее давление смеси газов.
Общее давление смеси газов, не вступающих друг с другом в химическое взаимодействие, равно сумме парциальных давлений газов, составляющих данную смесь:
| P = S pi. | (30) |
Если газ собран над жидкостью, то при расчетах его парциального давления следует иметь в виду, что оно равно разности общего давления и парциального давления пара жидкости, например, для газа, собранного над водой:
 . .
| (31) |
Закон эквивалентов
Эквивалентом вещества называется такое его количество, которое соединяется с 1 молем атомов водорода или замещает то же количество атомов водорода в химических реакциях. Эквивалентной массой Э называется масса одного эквивалента вещества. Для газов эквивалентным объемом называется объем, занимаемый при данных условиях одним эквивалентом вещества. Эквивалент (эквивалентную массу) можно вычислить по составу соединения данного элемента с любым другим, эквивалент (эквивалентная масса) которого известны пользуясь законом эквивалентов: массы взаимодействующих веществ пропорциональны их эквивалентным массам:
A + B ® C + D
 . .
| (32) |
На основе закона эквивалентов можно вывести следующие формулы для вычисления эквивалентных масс веществ:

| (33) |
где М – молярная масса элемента, оксида, кислоты, основания или соли, г/моль; Z – степень окисления элемента в продукте реакции, произведение числа атомов элемента и степени окисления элемента в оксидах, основность кислоты, кислотность основания, произведение числа атомов металла и степени окисления металла в соли.
Примеры решения задач
Пример 1. Определить массовую долю алюминия в его оксиде и вычислить, сколько алюминия теоретически можно выделить из боксита массой 15 т с содержанием Al2O3 87 %.
Решение. 1. Найдем молярную массу Al2O3:
 .
.
2. Примем количество вещества Al2O3 равным 1 моль, тогда количество вещества алюминия будет равно 2 моль. Масса оксида алюминия составит 102 г, а масса алюминия составит 2×27 = 54 г.
3. Вычислим массовую долю алюминия в его оксиде:
 .
.
4. Вычислим массу чистого Al2O3 в боксите:
 .
.
5. Масса алюминия, которую можно получить из боксита:
 .
.
Пример 2. При прокаливании 10 г некоторого вещества было получено 6,436 г CuO и 3,564 г CO2. Вывести формулу соединения.
Решение. 1. Найдем количество вещества оксида меди (II):

В 1 моль CuO содержится по 1 моль Cu и О, следовательно n (Cu) = n (O, CuO) = 0,081 моль.
2. Найдем количество вещества оксида углерода (IV):

В 1 моль CO2 содержится 1 моль Cu и 2 моль О, следовательно n (C) = 0,081 моль,
 = 2×0,081 = 0,162 моль.
= 2×0,081 = 0,162 моль.
3. Общее количество вещества кислорода n (O) = 0,081 + 0,162 = 0,243 моль.
4. Сопоставим количества вещества элементов между собой:
n (Cu): n (C): n (O) = 0,081:0,081:0,243 = 1:1:(0,243/0,081) = 1:1:3.
Полученные целые числа представляют собой стехиометрические индексы формулы вещества, химическая формула которого: CuCO3.
Пример 3. Соединение серы с фтором содержит 62,8 % серы и 37,2 % фтора. Данное соединение при объеме 118 мл в газообразном состоянии (температура 7°С, давление 96,34 кПа) имеет массу 0,51 г. Какова истинная формула соединения?
Решение. 1. Рассчитаем истинную молярную массу соединения по уравнению Менделеева – Клапейрона (26):  г/моль.
г/моль.
2. Обозначим x и y количество атомов серы и фтора в молекуле соответственно (S x F y). Зная процентное содержание каждого элемента в соединении и его молярную массу, получим 
3. Таким образом, простейшая формула соединения – SF. Его молярная масса: М = 32+19=51 г/моль.
Соотношение молярных масс, истинной и простейшей:






