Теоретическое введение
Электролитами называют вещества (кислоты, основания, соли), которые в растворах диссоциируют на ионы и проводят электрический ток.
Электролитическая диссоциация – распад молекул растворенного вещества на ионы под действием полярных молекул растворителя.
Кислоты – электролиты, диссоциирующие в растворах с образованием ионов водорода:
HNО2 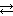 H+ + NО2‾.
H+ + NО2‾.
Основания – электролиты, диссоциирующие в растворах с образованием гидроксид-ионов:
NH4OH 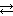 NH4+ + OH‾.
NH4+ + OH‾.
Существуют электролиты, которые могут диссоциировать как кислоты и как основания. Такие электролиты называются амфотерными. К ним относятся Be(OH)2, Zn(OH)2, Pb(OH)2, Sn(OH)2, Al(OH)3, Ga(OH)3, Cr(OH)3.
Диссоциацию растворимой части амфотерного электролита можно представить следующей схемой:
2H+ + BeO22− 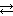 Be(OH)2
Be(OH)2 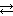 Be2+ + 2OH‾.
Be2+ + 2OH‾.
Соли – электролиты, которые при растворении в воде диссоциируют, отщепляя положительные ионы, отличные от ионов водорода, и отрицательные ионы, отличные от гидроксид-ионов:
Al2(SO4)3 → 2Al3+ + 3SO42‾;
средняя соль
NaHCO3 → Na+ + HCO3‾;
кислая соль
CuOHCl 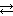 CuOH+ + Cl‾.
CuOH+ + Cl‾.
основная соль
Все электролиты делят на сильные и слабые. Сильные электролиты – это вещества, которые в водных растворах практически полностью диссоциируют на ионы. Сильными электролитами являются: все хорошо растворимые
соли, кислоты (H2SO4, HNO3, HCl, HBr, HI, HClO4), щелочи (LiOH, NaOH, KOH, RbOH, CsOH, Ca(OH)2, Sr(OH)2, Ba(OH)2).
Слабые электролиты – это вещества, которые в водных растворах не полностью диссоциируют на ионы. К слабым электролитам относятся: H2O, NH4OH; некоторые соли; кислоты CH3COOH, HF, HNO2, HCN, HClO, H2SO3, H2CO3, H2S, H3PO4; все нерастворимые в воде основания, например Mg(OH)2, Fe(OH)3, Cu(OH)2.
Реакции в растворах электролитов протекают между ионами. Обычно такие реакции изображаются при помощи ионно-молекулярных уравнений, порядок составления которых следующий:
а) записывают молекулярное уравнение реакции и в обеих частях уравнения подчеркивают вещества, которые не будут полностью диссоциировать на ионы (нерастворимые вещества, слабые электролиты, газы):
AgNO3 + KCl = AgCl ↓ + KNO3;
б) составляют полное ионное уравнение реакции. Осадки, газы и слабые электролиты полностью на ионы не диссоциируют, поэтому в ионных уравнениях записываются в молекулярном виде:
Ag+ + NO3‾ + K+ + Cl‾ = AgCl↓ + K+ + NO3‾;
в) составляют краткое ионное уравнение, сокращая одинаковые ионы с обеих сторон:
Ag+ + Clˉ = AgCl↓.
Реакции обмена в растворах сильных электролитов протекают до конца
или практически необратимо, когда ионы, соединяясь друг с другом, образуют вещества:
· нерастворимые (↓):
3CaCl2 + 2Na3PO4 = Ca3(PO4)2↓ + 6NaCl
3Ca2+ + 6Cl‾ + 6Na+ + 2PO43- = Ca3(PO4)2↓ + 6Na+ + 6Cl‾
3Ca2+ + 2PO43- = Ca3(PO4)2↓;
· газообразные (↑):
2HCl + Na2S = H2S ↑ + 2NaCl
2H+ + 2Cl‾ + 2Na+ + S2- = H2S↑ + 2Na+ +2Cl‾
2H+ + S2- = H2S↑;
· малодиссоциирующие (слабые электролиты):
H2SO4 + 2KNO2 = 2HNO2 + K2SO4
2H+ + SO42− + 2К+ + 2NO2‾ = 2HNO2 + 2K+ + SO42−
H+ + NO2‾ = HNO2.
В тех случаях, когда нет ионов, которые могут связываться между собой с образованием осадка, газа, слабого электролита, реакции обмена не протекают.
Нередко встречаются процессы, в уравнениях которых с одной стороны равенства имеется малорастворимое соединение, а с другой – слабый электролит. Такие реакции протекают обратимо, причем равновесие смещается в сторону наименее диссоциировааных веществ. Так, равновесие в системе
Mg(OH)2 ↓ + 2HCl 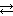 MgCl2 + 2H2O
MgCl2 + 2H2O
Mg(OH)2↓ + 2H+ + 2Cl‾ 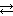 Mg2+ + 2Cl‾ + 2H2O
Mg2+ + 2Cl‾ + 2H2O
Mg(OH)2↓ + 2H+ 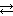 Mg2+ + 2H2O
Mg2+ + 2H2O
смещено вправо, в сторону малодиссоциированных молекул воды.
Примеры решения задач
Пример 11.1. Составить молекулярные уравнения реакций, которым соответствуют следующие ионно-молекулярные уравнения:
Fe(OH)3 + 3H+ = Fe3+ + 3H2O;
H3PO4 + 3OH− = PO43− + 3H2O;
HCO3− + OH− = CO32− + H2O.
Решение. В левой части данных ионно-молекулярных уравнений указаны ионы, которые образуются при диссоциации сильных электролитов, следовательно, при составлении молекулярных уравнений следует исходить из соответствующих растворимых сильных электролитов. Например:
Fe(OH)3 + 3HCl = FeCl3 + 3H2O;
H3PO4 + 3NaOH = Na3PO4 + 3H2O;
KHCO3 + KOH = K2CO3 + H2O.
При выполнении подобных заданий следует пользоваться табл. Б.3.
Пример 11.2. Составить молекулярные и ионно-молекулярные уравнения реакций, подтверждающие амфотерный характер гидроксида свинца.
Решение. Амфотерные электролиты могут диссоциировать по типу кислоты и основания, поэтому Pb(OH)2 может растворяться как в кислоте, проявляя свойство основания, так и в щелочи, проявляя свойства кислоты.
Как основание: Pb(OH)2 + 2HNO3 = Pb(NO3)2 + 2H2O
Pb(OH)2 + 2H+ = Pb2+ + 2H2O.
Как кислота: Pb(OH)2 + 2NaOH = Na2[Pb(OH)4]
Pb(OH)2 + 2OH‾ = [Pb(OH)4]2−.
Схема диссоциации Pb(OH)2:
2H+ + [Pb(OH)4]2− 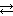 Pb(OH)2 + 2H2O
Pb(OH)2 + 2H2O 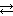 [Pb(H2O)2]2+ +2OH‾.
[Pb(H2O)2]2+ +2OH‾.






