–ассмотрим методику расчЄта pH в растворах гидролизующихс€ солей. –авновесные концентрации участников процесса гидролиза по каждой ступени (вода не учитываетс€) св€заны друг с другом через соответствующую константу гидролиза г, которую рассчитывают по следующему правилу:
константа гидролиза г равна частному от делени€ ионного произведени€ воды w = 10Ц14 на константу диссоциации а слабой кислоты ( b слабого основани€), которые образовались в результате гидролиза.
ѕример. —лаба€ фосфорна€ кислота диссоциирует по трем ступен€м:
H3PO4 → H2PO4Ц → HPO42Ц → PO43Ц
константы диссоциации Kа1 Kа2 Kа3
при этом образуютс€ три типа анионов, способных к гидролизу.
»он PO43Ц гидролизуетс€ по трем ступен€м и кажда€ из них имеет свою константу гидролиза: PO43- → HPO42- → H2PO4- → H3PO4. онстанта гидролиза: Kг1 = w / а3; Kг2 = w / а2; Kг3 = w / а1.
¬ качестве примера рассмотрим простейший случай расчета pH в растворе средней соли, например, фосфата натри€ с мол€рной концентрацией с0 моль/л.
Na3PO4 → 3Na+ + PO43Ц
с0 3с0 с0
ќбозначим степень гидролиза иона PO43Ц по первой ступени через h1 (h1<<1), тогда к моменту установлени€ равновеси€ подверглось гидролизу сгидр. (PO43Ц) = h1 Јс0 и
PO43Ц + H2O ↔ HPO42Ц + OHЦ
до гидролиза с0 моль/л ½ ê - ½ -
равновесие [PO43-] = с0 Ц сгидр. =½ ê [HPO42-] = h1 с0 ½ [OHЦ] = h1 с0
= с0 (1 Ц h1)
√идролизующийс€ ион PO43Ц образовалс€ по третьей ступени диссоциации фосфорной кислоты, поэтому г1 = 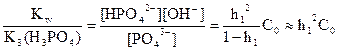 ,
,
откуда h1 = 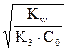 , [OHЦ] = h1 —0 =
, [OHЦ] = h1 —0 = 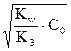 и pOH = - lg [OHЦ]= =(
и pOH = - lg [OHЦ]= =(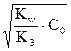 ), а pH = 14 Ц pOH.
), а pH = 14 Ц pOH.
«аметим, что использование упрощенной формулы (1 << h1) возможно, если константа гидролиза г < 10Ц3, концентраци€ иона с0 > 0,001 моль/л; в противном случае следует проводить вычислени€ по общей формуле.
–асчет рЌ в растворах кислых солей более сложен, поскольку анион может участвовать в двух конкурирующих процессах Ц гидролиза и диссоциации. “ем не менее, можно легко определить характер раствора, сравнива€ константы равновеси€ этих процессов, и преобладает тот из них, у которого константа больше.
ѕример Ц в растворе гидрофосфата натри€ Na2HPO4 → 2 Na+ + HPO42Ц, причем ион HPO42Ц может далее
а) диссоциировать по III ступени HPO42Ц ↔ PO43Ц + Ќ+; 3(Ќ3–ќ4) = 1,26Ј10Ц12
б) гидролизоватьс€ HPO42Ц + H2O ↔ H2PO4Ц + OHЦ г = w / 2(Ќ3–ќ4) =
= 10Ц14 / 6,34Ј10Ц8 = 1,57Ј10Ц7.
¬идно, что преобладает процесс гидролиза HPO42Ц и раствор данной соли слабощелочной.
ќЅ”„јёў»≈ «јƒј„»
1. ¬ычислить константу гидролиза г, степень гидролиза h и рЌ раствора хлорида аммони€ с концентрацией соли с(NH4Cl)=0,01 моль/дм3.
–ешение:
1) соль NH4Cl образована сильной кислотой HCl и слабым основанием NH4OH Ц гидролиз по катиону; гидролиз соли Ц процесс обратимый.
NH4Cl + H2O = NH4OH + HCl
NH4+ + H2O Û NH4OH + H+ - в результате гидролиза образуютс€ ионы Ќ+, т.е. среда в растворе кисла€.
2) константу гидролиза г рассчитывают по формуле:
|
|
|
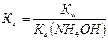 ,
,
где w Ц ионное произведение воды, w =10-14 (25 0—); b (NH4OH) Ц константа ионизации основани€ (справочна€ величина), b (NH4OH)=1,74Ј10-5.
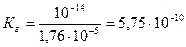
3) степень гидролиза h соли рассчитывают по формуле:
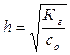 ,
,
где c о Ц мол€рна€ концентраци€ соли в растворе.
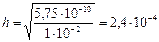
4) концентраци€ Ќ+ ионов равна концентрации гидролизованной части соли и ее определ€ют по формуле:
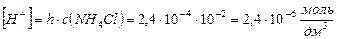
5) 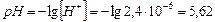
ќтвет: константа гидролиза соли NH4Cl равна 5,75Ј10-10; степень гидролиза составила 2,4Ј10-4; рЌ раствора равен 5,62.
2. ќпределить константу гидролиза, степень гидролиза и рЌ раствора ацетата кали€, если концентраци€ с (—Ќ3—ќќ )=0,1моль/дм3, а а (—Ќ3—ќќЌ)=1,8Ј10-5.
–ешение:
1) соль CH3COOK образована слабой кислотой CH3COOH и сильным основанием KOH Ц гидролиз по аниону, среда в результате гидролиза щелочна€:
CH3COOK + H2O = CH3COOH + KOH;
—H3COO- + H2O Û CH3COOH + OH- Ц накапливаютс€ ионы ќЌ-, среда щелочна€.
2) константу гидролиза г рассчитывают по формуле:
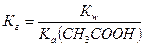 ,
,
где а Ц константа ионизации кислоты. 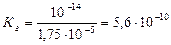
2) степень гидролиза h соли рассчитывают по уравнению:
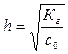 ,
,
где с0 Ц концентраци€ соли в растворе.
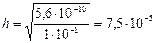
3) концентраци€ ќЌ- - ионов равна концентрации гидролизованной части соли:
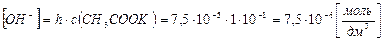
4) 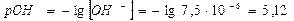
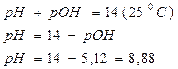
ќтвет: константа гидролиза соли CH3COOK равна 5,6Ј10-10; степень гидролиза составила 7,5Ј10-5; рЌ раствора 8,88.
3. ¬ результате реакции гидролиза гидрокарбоната натри€ в его растворе создаетс€ слабощелочна€ среда. –ассчитайте рЌ раствора, содержащего 10 г гидрокарбоната натри€ в 200 см3 раствора, если степень гидролиза равна 0,01 %.
–ешение:
1) соль NaHCO3 Ц кисла€ соль слабой угольной кислоты и сильного основани€ Ц гидролиз по аниону:
NaHCO3 + H2O = NaOH + H2O + CO2
HCO3- + H2O Û OH- + H2O + CO2 Ц среда щелочна€
2) концентраци€ ќЌ- - ионов равна концентрации гидролизованной части соли:
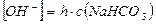 , где h Ц степень гидролиза соли, а с(NaHCO3) Ц мол€рна€ концентраци€ соли в растворе:
, где h Ц степень гидролиза соли, а с(NaHCO3) Ц мол€рна€ концентраци€ соли в растворе:
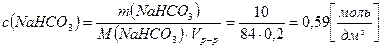 ,
,
тогда 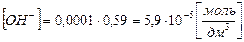
3) 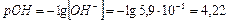
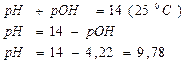
ќтвет: рЌ = 9,78.






