–абота пылеулавливающих аппаратов основана на: - гравитационное осаждение под действием сил т€жести Ц осаждение под действием центробежных сил Ц инерционное осаждение Ц зацепление(если рассто€ние от частицы, движущейс€ вместе с газовым потоком до обтекаемого тела, равно ее радиусу или меньше его) Ц диффузионное осаждение Ц электрическое осаждение (при ионизации газа, частицы осаждаютс€ на электродах)
јппараты пылеулавливани€: * механические: - циклоны Ц вихревые Ц ротационные Ц радиальные * гидравлические: центробежные Ц механические Ц турбулентные Ц скрубберы Ц пенные * ‘ильтрационные: - тканевые фильтры Ц зернистые Ц волокнистые.
ћетод конденсации: примен€ют дл€ улавливани€ паров и летучих растворителей. ¬ основе метода лежит €вление уменьшени€ давлени€ насыщенного пара растворител€ при понижении температуры. ƒостоинства: простота аппаратурного оформлени€ и эксплуатации установки. Ќедостатки: взрывоопасность процесса, высокие расходы холодильного реагента и электроэнергии, низкий вывод растворителей.
ћетод компримировани€ базируетс€ на том же €влении, что и метод конденсации, но применительно к парам растворителей, наход€щихс€ под высоким давлением. Ќедостатки: сложность аппаратурного выполнени€, невозможность работы с парами с низкой концентрацией.
57. јбсорбционные методы очистки газов(SO(2), N(x)O(y), H(2)S)
—уть абсорбции заключаетс€ в поглощении удал€емых компонентов жидкостью. ¬ зависимости от особенностей взаимодействи€ поглотителей и извлекаемого из газовой смеси компонента абсорбционные методы дел€тс€ на физическую и химическую абсорбцию. ƒл€ физической абсорбции примен€ют поглотители: воду, органические растворители, не вступающие в реакцию с извлекаемыми газами. ѕри химической абсорбции извлекаемые компоненты вступают в химическую реакцию с хемосорбентами, в качестве которых используют растворы минеральных и органических веществ, суспензии и органические жидкости.
»звестн€ковые и известковые методы
ќчистка от SO(2).
јбсорбци€ SO(2) сульфитом натри€: метод двухстадийный -
---Na(2)SO(3) + SO(2) + H(2)O 2NaHSO(3) ---
--- 2NaHSO(3) SO(2) + H(2)O + Na(2)SO(3) ---
¬тора€ стади€ Ц регенераци€ сульфата натри€ Ц проводитс€ при температуре 130 гр, при этом выдел€ютс€ газообразный S)(2). ќхлажденный раствор сульфата натри€ снова возвращаетс€ на абсорбцию, а SO(2) направл€етс€ на переработку в серную кислоту.
јммиачный способ улавливани€ SO(2):
--- SO(2) + NH(4)OH = NH(4)HSO(3) ---
--- (NH(4))(2)SO(3) + SO(2) + H(2)O = 2NH(4)HSO(3) --- при нагревании бисульфат аммони€ разлагаетс€:
--- 2NH(4)HSO(3) (NH(4))(2)SO(3) + SO(2) + H(2)O ---
¬ысока€ степень улавливани€ SO(2).
ћагнезиальные методы.
ƒиоксид серы поглощаетс€ суспензией оксиды-гидроксиды магни€. ¬ процессе хемосорбции образуютс€ кристаллогидраты сульфата магни€, которые сушат, а затем термически разлагают на SO(2) Ц содержащий газ и оксид магни€. √аз перерабатывают в серную кислоту, а оксид магни€ возвращают в абсорбцию. –еакции в абсорбере:
|
|
|
--- MgO + SO(2) = MgSO(3)--- MgSO(3) + SO(2) + H(2)O = Mg(HSO(3))(2)---
Ѕисульфат магни€ нейтрализуетс€ добавкой соответствующего количества свежего оксида магни€:
---Mg(HSO(3))(2) + MgO = 2MgSO(3) + H(2)O --- ќсадок подвергаетс€ термической обработке (800 Ц 900гр.) ---MgSO(3)MgO + SO(2)---
ќксид магни€ возвращаетс€ на абсорбцию,SO(2) перерабатываетс€ в серную кислоту или в серу. ‘осфатный метод Ц абсорбци€ SO(2) водным раствором фосфата натри€. ислотно-каталитический Ц применение разбавленной H(2)SO(3) в качестве катализаторов. ќзоно-каталитический. –адиционно-каталитический - использование органических сорбентов.
ќчистка от газов и оксидов азота.
ѕроблема Ц низка€ химическа€ активность и растворимость оксидов азота. ƒл€ абсорбции используют воду, растворы щелочей, кислоты и окислители.
јбсорбци€ водой: в газовую фазы выдел€етс€ часть менее опасного оксида азота, скорость окислени€ которого мала:
--- 3NO(2) + H(2)O →< 2HNO(3) + NO + Q --- ”тилизаци€ оксидов:
--- NO + H(2)O(2) → NO(2) + H(2)O ---
--- 3NO(2) + H(2)O → 2HNO(3) + NO---
--- N(2)O(3) + H(2)O(2) → N(2)O(4) + H(2)O---
--- H(2)O(4) + H(2)O → HNO(3) + HNO(2)---
јбсорбци€ щелочами: –еакции хемосорбции:
--- N(2)O(3) + NaCO(3) = 2NaNO(2) + CO(2) + Q ---
--- N(2)O(3) + Ca(OH)(2) = Ca(NO(2))(2) + H(2)O ---
--- 2NO(2) + Na(2)CO(3) = 2NaNO(2) + H(2)O ---
—елективные абсорбенты.
ƒл€ очистки газов при отсутствии кислорода используют растворы FeSO(4), FeCl(2), Na(2)SO(3), Na(2)S(2)O(3)Е ѕротекают следующие реакции:
--- FeCl(2) + NO → < Fe(NO)Cl(2) --- FeSO(4) + NO →< Fe(NO)SO(4)---
ѕрименение растворов восстановителей:
--- 2Na(2)S(2)O(3) + 6NO = 3Na(2)SO(4) + 2SO(2)---
ќчистка газов от сероводорода. √азы содержащие H(2)S Ц коррозийно-активны. ћышь€ково-щелочные методы. ѕримен€ют водный раствор кальцинированной соды и мышь€ка, содержащий 10 Ц 18 г/л мышь€ковистого ангидрида As(2)O(3) в виде Na(3)AsO(4), Na(2)HasO(3)Еобразование поглотительного раствора:
--- 2NaHAsO(3) + 5H(2)S →< Na(4)As(2)S(5) + 6H(2)O ---
Na(4)As(2)S(5) + O(2) →< Na(4)As(2)S(5)O(2) ---
Ba(4)As(2)S(5)O(2) + H(2)S = Na(4)As(2)S(6)O + H(2)O---
--- Na(4)As(2)S(6)O + H(2)S = Na(4)As(2)S(7) + H(2)O ---
—тепень очистки 96 Ц 97%.
јбсорбци€ этаноламинами.
ƒл€ извлечени€ H(2)S используют моно- и триэтаноламины. ѕроцесс поглощени€:
2(OH-CH(2)-CH(2)-NH(2)) + H(2)S =
= OH-CH(2)-CH(2)-NH(3)\S
-- OH-CH(2)-CH(2)-NH(3)\S + H(2)S =
OH-CH(2)-CH(2)-NH(3)/
-- OH-CH(2)-CH(2)-NH(3)/
= 2(OH-CH(2)-CH(2)-NH(3)-HS)
ѕри 25-40 гр. Ќаправление реакции поглощени€ слева направо, с повышением температуры до 105 гр. Ц обратное направление с удалением из раствора H(2)S и CO(2).
‘осфатный метод.
ƒл€ поглощени€ H(2)S используетс€ 40-50% растворы фосфата кали€:
K(3)PO(4) --- K(3)PO(4) + H(2)S < KHS + K(2)HPO(4) ---
»з растворов сероводород удал€етс€ кип€чением.
58. —уть адсорбционных методов очистки газов. “ипы адсорбентов.
јдсорбционные методы очистки основаны на поглощении примесей твердыми телами с развитой поверхностью, адсорбентами. ѕоглощаемые молекулы удерживаютс€ на поверхности твердых тел силами
¬ан-дер-¬аальса(физическа€ адсорбци€) или химическими силами(хемосорбци€). —тадии адсорбции: - перенос молекул газа к внешней поверхности твердого тела Ц проникновение молекул газа в поры твердого тела Ц собственно адсорбци€. јдсорбци€ рекомендуетс€ дл€ очистки газов с невысокой концентрацией вредных компонентов. јдсорбированные вещества удал€ют из адсорбентов с помощью десорбции инертным газом или паром. ѕреимущество: высока€ степень очистки. Ќедостатки: УчистыеФ (сухие и без пыли) газы, небольша€ скорость. јдсорбенты Ц материалы высокоразвитой внутренней поверхностью(природные и синтетические): активированные угли, силикагели, алюмогели, цеолиты, иониты. јктивированные угли Ц гидрофобны. ƒл€ адсорбции газов и паров используют микропористые гранулированные активированные угли. —иликагели Ц гидратированные аморфные кремнеземы (SiO(2)nH(2)O), €вл€ющиес€ реакционно-способными соединени€ми переменного состава, превращени€ которых идет по механизму полконденсации. «азоры м/у частицами образуют пористую структуру селикагел€. ѕолучают путем осаждени€ аморфного кремнезема из силикатно-щелочных металлов. —лужат дл€ поглощени€ пол€рных веществ. јлюмогели (Al(2)O(3)*nH(2)O где 0< n < 6) Ц получают прокаливанием различных гидроксидов алюмини€. »спользуют дл€ улавливани€ пол€рных органических соединений и осушки газов. ÷еолиты - алюмосиликаты, содержащие в своем составе оксиды щелочных и щелочноземельных металлов. ’арактеризуютс€ регул€рной структурой пор, соизмеримых с размерами молекул. ќбща€ химическа€ формула: Me(2/n)C*Al(2)O(3)*xSi(2)*yH(2)O, где Me катион металла, n Ц его валентность. ѕолучают синтетически и добывают при разработки месторождений. ќбладают наибольшей адсорбцией по парам пол€рных соединений и веществ с кратными св€з€ми в молекулах. »ониты - высокомолекул€рные соединени€ с развитой поверхностью.
|
|
|
аталитические методы.
св€занны с химическим превращением токсичных компонентов в нетоксичные в присутствии катализаторов. јппараты Ц реакторы различной конструкции. »спользуютс€ дл€ очистки от: оксидов азота, серы, углерода и от органических примесей.
ќксид азота восстанавливаетс€ газом Ц восстановителем(CO,CH(4)Е) в присутствии катализаторов. ¬ качестве катализаторов используют различные металлы, которыми покрывают огнеупорные материалы (носители). „асто примен€ют палладиевый катализатор, нанесенный на оксид алюмини€. “емпература 400 470 гр. –еакции: --- 4NO + CH(4) = CO(2) + 2H(2)O + 2N(2) --- 2NO(2) + CH(4) = CO(2) + 2H(2)O + N(2) --- 2NO + 2H(2) = N(2) + 2H(2)O --- 2NO(2) + 4H(2) = N(2) + 4H(2)O --- 2NO + PCO → N(2) + 2CO(2) --- 2NO(2) + 4CO → N(2) + 4CO(2)---
ќчистка от оксида углерода €вл€етс€ наиболее рациональной. ѕроцесс гидрировани€ оксида углерода на никелевых и железных катализаторах провод€т при высоких давлени€х и повышенных температурах: --- CO + 3H(2) = CH(4) + H(2)O --- C)(2) + 4H(2) = CH(4) + 2H(2)O --- 1/2O(2) + H(2) = H(2)O---
ќчистка от диоксида серы Ц основана на принципе окислени€ SO(2) и SO(3) контактным методом. »спользуют метод очистки с получением сульфата аммони€, который можно использовать как удобрение. SO(2) окисл€ют до SO(3) в присутствии V(2)O(5) при 450 Ц480 гр. «атем при температуре 220-260 гр. ¬вод€т газообразный аммиак. ѕолученные кристаллы сульфата аммони€ отдел€ют в циклонах и электрофильтрах.
аталитическа€ очистка газов от органических веществ. ¬ качестве катализаторов используют Cu, Cr, Co, Mn, Ni Е в отдельных случа€х бокситы, цеолиты. атализаторы условно дел€тс€ на: - цельнометаллические (металлы платиновой группы или неблагородные металлы, нанесенные на ленты, сетки, спирали из нержавеющей стали) Ц смешанные (металлы платиновой группы или оксиды неблагородных металлов, нанесенные на оксид алюмини€, нержавеющую сталь) Ц керамические (-=-, нанесенные на керамическую основу в виде сот или решеток) Ц насыпные (гранулы или таблетки из оксида алюмини€ с нанесенными на него металлами платиновой группы или оксидами неблагородных металлов, зерна оксидов небл. ћет.) ѕреимуществом обладают катализатор, нанесенные на мет носители. ќни более термостабильны, прочны, с легкой регенерацией.
|
|
|
ќчистка газов от серо-органических соединений заключаетс€ в их окислении при повышенных температурах. аталитическое окисление производ€т кислородом, образованием кислородных соединений серы. ¬ качестве катализаторов процессов гидрировани€ серо-органических соединений водородом используют контактные массы на основе оксидов Fe, Co, Ni, Cu, Zn. ѕри гидрировании вод€ным паром используют катализаторы, содержащие в качестве компонента оксид железа.
«адача. онцентраци€ растворимого соединени€ ј в сточной воде составл€ет —ј. ¬о сколько раз необходимо разбавить эту воду, чтобы можно было сливать ее в канализацию, если известна ѕƒ соединени€ ј?
 —нд=0,0005 моль/л m=νM
—нд=0,0005 моль/л m=νM
ѕƒ нд=0,0005мг/л mнд=0,0005*200=0,1 г

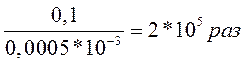
 —NO3-=0.01 моль/л m=νM
—NO3-=0.01 моль/л m=νM
ѕƒ NO3-=45мг/л m NO3-=0,01*62=0,62 г

 —NO2-=0,5 моль/л m=νM
—NO2-=0,5 моль/л m=νM
ѕƒ NO2-=3,3мг/л m NO3-=0,5*46=23 г
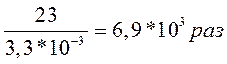
«адача. ¬ воздухе обнаружено присутствие примесей ј, ¬, — в концентраци€х —ј, —¬, ——. —оотв. ли установленным нормативам качество воздуха, если ѕƒ этих веществ равны соотв. ѕƒ ј, ѕƒ ¬, ѕƒ —? ѕри ответе учесть эффект суммации.
находитс€ по формуле: 
если ≤1, то соответствует, если неравенство не выполн€етс€, значит концентраци€ в воздухе вредных примесей превышает нормам.
«адача. ∆есткость некоторого образца воды обусловливаетс€ только дикарбонатом магни€. ѕри кип€чении 0,5 л воды в осадок выпало 14 мг Mg(OH)2. „ему равна жесткость воды?
–ешение 1.
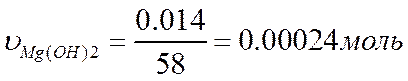 , или
, или
 эквивалентной массы, или
эквивалентной массы, или
0,48 мэкв Mg(OH)2
¬ 0,5 л воды содержитс€ 0,48 мэкв Mg(OH)2,
жесткость воды равна 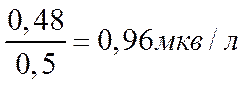
–ешение 2.
¬ 1 л воды содержитс€ 0,014/0,5 = 0,028 г Mg(OH)2,
что составл€ет 0,028/0,029=0,00096 г-экв
или 0,96 мг-экв (29 Ц эквивалент Mg(OH)2)
—ледовательно, жесткость воды 0,96 мг-экв
«адача. „ему равна жесткость (в ммоль/л) 0,003 ћ раствора MgCl2?
0,003 ћ MgCl2
0,003 ћ = 0,003 моль/л = 3ммоль/л
«адача. Ќа титрование 0,5 л образца воды израсходовано 22,8 мл 0,1 н Ќ—1. „ему равна карбонатна€ жесткость воды?
 VH2O=0.5 л 0,1н HCl
VH2O=0.5 л 0,1н HCl
VHCl=22,8 мл 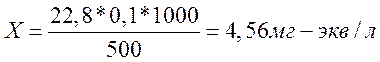
«адача. —колько л 20 % сол€ной кислоты, плотностью 1,098 г/см3, потребуетс€ дл€ нейтрализации 700л 5 ћ щелочной сточной воды?
1 л раствора имеет массу 1098 г и содержит 1098*0,20=219,60 г HCl, что составл€ет
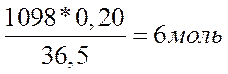
C1V1 = C2V2
700л*5ћ=’л*6ћ

«адача. –ассчитайте минимальный объем (в литрах) 15 % раствора соды (плотность 1,158 г/мл), необходимый дл€ устранени€ жесткости 100 л природной воды с содержанием гидрокарбонат-ионов, равным 0,61 г/л.
1 л раствора имеет массу 1158 г и содержит 1158*0,15=173,7 г Na2CO3
C1V1 = C2V2
100*0,61=’*173,7

«адача. ƒиоксид углерода поглощают раствором гидроксида кальци€. ¬начале образуетс€ осадок, затем он исчезает. ќпределите общий объем газа (в литрах, н.у.), израсходованный при образовании 74 г осадка.
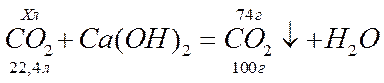
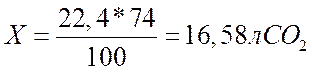
«адача. —месь бромидов натри€ и кали€ примен€ют в медицине как успокоительное средство. Ќайдите число бромид-ионов, поступивших в организм после приема 10 мл раствора, содержащего по 30 г бромида натри€ и бромида кали€ в 1 л.
ν=30/103=0,29 моль NaBr
ν=30/119=0,25 моль KBr
[Br]-общ=0,29+0,25=0,54 моль/л
0,54 моль Ц 1000 мл
’ Ц 10 мл
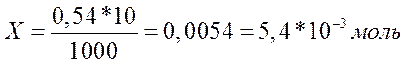
число Br-=5.4*10-3=32.5*1020
«адача. √азова€ смесь содержит —ќ2, —ќ, ќ2 и N2. ѕри анализе 100 мл смеси методом химического поглощени€ получены следующие результаты.
| омпонент | оличество газа после поглощени€, мл |
| —ќ2 | 83,20 |
| ќ2 | 82.40 |
| —ќ | 75.60 |
| N2 | 75,60 |
ќпределите количественный состав смеси (объемный %), если происходит последовательное поглощение газов из смеси, a N2 не поглощаетс€.
|
|
|
VCO2=83,20 мл
Vќ2=82,40 мл
V—ќ=75,60 мл
VN2=75,60 мл
Vобщ=83,20+82,40+75,60+75,60=316,80 мл
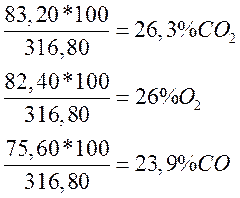
100-(26,3+26+23,9)=23,8% N2






