| ќпределение | ÷иклоалканы (циклопарафины) Ч насыщенные ”¬, содержащие цикл из трех и более атомов углерода. ќтнос€тс€ к алициклическим ”¬ |
| ќбща€ формула | CnH2n (n ≥ 3) |
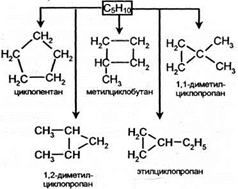
    ѕростейшие пред≠ставители (без боковых цепей)
»зомери€ и номенклатура ѕростейшие пред≠ставители (без боковых цепей)
»зомери€ и номенклатура
|
циклопропан циклобутан циклопентан циклогексан
»зомери€ цепи
|
ћежклассова€ изомери€. ÷иклоалканы изомерны алкенам
 циклопропан
циклопропан
|
‘изические свойства
 |  | ||||||
 |  | ||||||
¬ысшие циклоалканы
(C3H6) (C4H8) (C5H10) (C6H12)
Ѕ/цв. газы ∆идкости ∆идкости
ѕлохо растворимы в воде.
’имические свойства
ћалые циклы (—3, —4) неустойчивы и легко разрушаютс€. ќбычные циклы (—5Ч—7) очень устойчивы и не склонны к разрыву в химических реакци€х. ƒл€ малых циклов характерны реакции присоединени€ (сходство с алкенами); дл€ обычных циклов Ч реакции замещени€ (сходство с алканами).
| “ип, название реакции | ѕримеры реакций |
| I. –еакции присоединени€ 1. √идрирование (образуютс€ алканы) |  Ni
+ + Ќ2 Ѓ —Ќ3-—Ќ2-—Ќ3
циклопропан пропан Ni
+ + Ќ2 Ѓ —Ќ3-—Ќ2-—Ќ3
циклопропан пропан
|
| 2. √алогенирование (образуютс€ дигалогеналканы) |  + Br2 Ѓ BrЦCH2ЦCH2ЦCH2ЦBr
÷иклопропан 1,3-дибромпропан
+ Br2 Ѓ BrЦCH2ЦCH2ЦCH2ЦBr
÷иклопропан 1,3-дибромпропан
|
| 3.√идрогалогени-рование (образуютс€ галогеналканы) |
 + HBr Ѓ CH3ЦCHЦCH2ЦCH2
| |
Br H
метилциклопропан 2-бромбутан + HBr Ѓ CH3ЦCHЦCH2ЦCH2
| |
Br H
метилциклопропан 2-бромбутан
|
| II. –еакции замещени€ √алогенирование (образуютс€ галогенциклоалканы) | hv
 + Cl2 Ѓ + Cl2 Ѓ  + HCl
циклогексан хлорциклогексан + HCl
циклогексан хлорциклогексан
|
| III. –еакции дегидрировани€ (образуютс€ ароматические ”¬) |  Pd,300C
Ѓ —6Ќ6 + 3H2
циклогексан бензол Pd,300C
Ѓ —6Ќ6 + 3H2
циклогексан бензол
|
—пособы получени€
| Ќазвание способа | ”равнени€ реакций |
| 1. ÷иклизаци€ дигалогеналканов | H2C Ц CH2 Ц Cl H2C Ц CH2 | + 2Na Ѓ | | + 2NaCl H2C Ц CH2 Ц Cl H2C Ц CH2 1,4-дихлорбутан циклобутан |
     2. √идрирование циклоалкенов или ароматических ”¬ 2. √идрирование циклоалкенов или ароматических ”¬
| + H2 Ѓ циклопентен циклопентан T,kat + 3H2 Ѓ бензол циклогексан |
Ќепредельные (ненасыщенные) углеводороды.
јлкены (этиленовые углеводороды)
| ќпределение | јлкены (олефины) Ч это ”¬ нецикличе≠ского строени€, в молекулах которых два ато≠ма углерода наход€тс€ в состо€нии sp2-гибридизации и св€заны друг с другом двой≠ной св€зью (длина св€зи  0,133 нм) 0,133 нм)
|
| ќбща€ формула | CnH2n (n ≥ 2) |
| √омологический р€д, изомери€, номенклатура | 1. CH2 = CH2 этен(этилен); радикал CH2 = CHЦ винил
2. CH2 = CH Ц CH3 пропен (пропилен);
радикал CH2 = CH Ц CH2 Ц аллил
3.C4H8:
CH2 = CH Ц CH2 Ц CH3 бутен-1 (бутилен)
 цис-бутен-2 цис-бутен-2
 транс- бутен-2
CH2 = C Ц CH3
| 2-метилпропен-1 (изобутилен)
CH3
“ипы изомерии:
а) изомери€ цепи;
б) изомери€ положени€ двойной св€зи;
в) цис, транс-изомери€;
г) межклассова€ изомери€ (см. Ђ÷иклоалканыї). транс- бутен-2
CH2 = C Ц CH3
| 2-метилпропен-1 (изобутилен)
CH3
“ипы изомерии:
а) изомери€ цепи;
б) изомери€ положени€ двойной св€зи;
в) цис, транс-изомери€;
г) межклассова€ изомери€ (см. Ђ÷иклоалканыї).
|
|
|
|
‘изические свойства
—2Ќ4, —3Ќ6, —4Ќ8 —5Ќ10 Е —16Ќ32 C17H34 Е
√азы ∆идкости “вердые вещества
ѕлохо растворимы в воде.
’имические свойства
’имические свойства алкенов определ€ютс€ наличием в их молекулах двойной св€зи. ¬ход€ща€ в ее состав π-св€зь легко разруша≠етс€, и по месту разрыва могут присоедин€тьс€ различные реагенты. “аким образом, дл€ алкенов наиболее характерны реакции присоеди≠нени€:
| |
 +XY Ѓ Ц — Ц C Ц
+XY Ѓ Ц — Ц C Ц
| |
X Y
| “ип, название ре≠акции | ѕримеры реакций |
| I. –еакции присоединени€ 1. √идрирование (образуютс€ алканы) | T,Ni —Ќ2=—ЌЧ—Ќ3 + Ќ2 Ѓ —Ќ3Ч—Ќ2Ч—Ќ3 пропен пропан |
| 2. √алогенирование (образуютс€ дигалогеналканы) | —Ќ2=—Ќ2 + Cl2 Ѓ ClЦCH2ЦCH2ЦCl этен 1,2-дихлорэтан —Ќ3Ц—Ќ=—ЌЦ—Ќ3+ ¬г2 Ѓ —Ќ3Ц—ЌЦ—ЌЦ—Ќ3 бутен-2 (H2O) | | Br Br 2,3-дибромбутан |
| –еакци€ с бромной водой (р-р ¬г2 в Ќ2ќ) €вл€етс€ качественной реакцией на алкены и другие непредельные ”¬ (бромна€ вода обес≠цвечиваетс€) | |
| 3. √идрогалогенирование (обра≠зуютс€ галогеналканы) | —Ќ2=—Ќ2 + HCl Ѓ —Ќ3ЦCH2ЦCl этен хлорэтан Br | —Ќ2=—ЌЦ—Ќ3 + Ќ¬г Ѓ —Ќ3Ц—ЌЦ—Ќ3 пропен 2-бромпропан ѕоследн€€ реакци€ протекает в соответст≠вии с правилом ћарковникова: при присое≠динении молекул типа Ќ’ к несимметричным алкенам атом водорода присоедин€етс€ к более гидрогенизированному атому углерода двойной св€зью |
| 4. √идратаци€ (образуютс€ пре≠дельные одно≠атомные спирты алканолы) | T,H+ —Ќ2=—Ќ2 + ЌќЌ Ѓ CH3ЦCH2ЦOH этен этанол T,H+ —Ќ2=—ЌЦ—Ќ2Ц—Ќ3 + ЌќЌ Ѓ CH3ЦCHЦCHЦCH3 бутен-1 | OH бутанол-2 |
| II. –еакции окислени€ 1. √орение | T —2Ќ4 + 3ќ2 Ѓ 2—ќ2 + 2Ќ2ќ |
| 2. Ќеполное ката≠литическое окис≠ление | Ag,2000C
2—Ќ2=—Ќ2 + O2 Ѓ 2  этиленоксид
этиленоксид
|
| 3. ќкисление перманганатом кали€ в нейтральной или щелочной среде (образуютс€ двух≠атомные спирты-гликоли) Ч реак≠ци€ ¬агнера | 3CH2=CH2+2KMnO4+ 4H2OЃ3CH2ЦCH2+2KOH+2MnO2¯ этилен | | OH OH этиленгликоль или упрощенно: CH2ЦCH2 + (O) + H2O Ѓ CH2ЦCH2 | | OH OH »спользуетс€ как качественна€ реакци€ на алкены и другие непредельные ”¬ (р-р ћnќ4 обесцвечиваетс€) |
| 4. ќкисление перманганатом кали€ в кислой среде (образуютс€ карбоновые кислоты) Ч окислительное расщепление двой≠ной св€зи | H+
CH3ЦCH=CHЦCH3 + 4(O) Ѓ 2  бутен-2 (из KMnO4) уксусна€ кислота
H+
CH2=CHЦCH3 + 4(O) Ѓ
бутен-2 (из KMnO4) уксусна€ кислота
H+
CH2=CHЦCH3 + 4(O) Ѓ 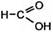 + +  пропен (из KMnO4) муравьина€ уксусна€ ки слоты
пропен (из KMnO4) муравьина€ уксусна€ ки слоты
|
| III. –еакции полимеризации | T,P,kat n CH2=CH2 Ѓ (ЦCH2ЦCH2Ц)n этилен(мономер) полиэтилен(полимер) T,P,kat n CH2=CHЦCH3 Ѓ (ЦCH2ЦCHЦ)n пропилен | CH3 полипропилен |
—пособы получени€
| Ќазвание способа | ”равнени€ реакций |
| 1. ƒегидрирование алканов | T,P,kat CnH2n+2 Ѓ CnH2n + Ќ2 |
| 2. √идрирование алкинов | Ni CnH2n-2 + Ќ2 Ѓ CnH2n |
| 3. рекинг алканов | —м. Ђјлканыї |
| 4. ƒегидратаци€ спиртов (алканолов) | H2SO4(конц.),~1700C CH3ЦCH2ЦOH Ѓ CH2=CH2 + H2O этанол этен H2SO4(конц.),~1700C CH3ЦCHЦCH2ЦCH3 Ѓ CH3ЦCH=CHЦCH3 + H2O | OH бутанол-2 бутен-2 ѕоследн€€ реакци€ протекает в соответст≠вии с правилом «айцева: при отщеплении молекул типа Ќ’ атом водорода отрыва≠етс€ от менее гидрогенизированного сосед≠него атома углерода |
| 5. ƒегидрогало-генирование гало-геналканов под действием спирто≠вого р-ра щелочи | “ CH3ЦCH2ЦCl + KOH Ѓ CH2=CH2 + KCl + H2O хлорэтан (спирт. р-р) этен “ CH3ЦCH2ЦCHЦCH3+NaOH Ѓ CH3ЦCH=CHЦCH3+NaBr+H2O | (спирт. р-р) Br 2-бромбутан бутен-2 |
| 6. ƒегалогенирование дигалогеналканов под дей≠ствием магни€ или цинка | “ CH3ЦCH2ЦCHЦCH2+Mg ЃCH3ЦCH2ЦCH=CH2 + MgBr2 | | Br Br 1,2-дибромбутан бутен-1 (2 атома галогена должны находитьс€ у соседних атомов углерода.) |
|
|
|






