Опыт №1а Получение слабой кислоты.
Кд>1 Кд>1 Кд<1 Кд>1
CH3COONa + HCl = CH3COOH + NaCl
ацетат натрия соляная кислота уксусная кислота хлорид натрия
При проведении реакции отметим выделение запаха уксуса, что означает появление уксусной кислоты в продуктах реакции.
CH3COO- + Na + + H+ + Cl - = CH3COOH + Na + + Cl -
CH3COO- + H+ = CH3COOH (уксусная кисллота)
Кд(CH3COOH) <1 – слабая кислота = 1*107
Кд(HCl)>1 - сильная кислота = 1,85*10-5
Вывод: При взаимодействии соли с кислотой можно получить слабую соль. Слабый электролит имеет Кд<1; Кд(CH3COOH) <1.
Опыт №2б Смещение равновесия диссоциации слабого основания.
1. NH4OH + ф-ф (Окраска раствора стала малиновой (есть гидроксид аммония), что говорит о наличие щелочной среды)
2. NH4OH + ф-ф + NH4Cl (Окраска стала розовой)
I) NH4OH↔ NH4+ + OH- II) NH4Cl↔ NH4+ + Cl-
Кд = 1,7*10-5 < 1
Вывод: При добавлении к слабому основанию (NH4OH) хлорида аммония произошло смещение равновесия диссоциации, так как цвет раствора стал более светлым.
Кд(NH4OH) < 1 Кд(NH4OH) = [NH4+][ OH-]/[NH4OH]
Опыт №3 Реакции нейтрализации.
1. NaOH + ф-ф + СН3СООН→СН3СООNa + H2O
гидроксид натрия уксусная кислота ацетат натрия вода
2. NaOH + ф-ф + HCl ↔ NaCl + H2O
соляная кислота хлорид натрия
При добавлении к NaOH индикатора ф-ф пронаблюдали малиновую окраску раствора (так как щелочная среда)
Оба раствора обесцвечены, так как среда стала нейтральной.
Кд>1 Кд<1 Кд>1 Кд<1
1. NaOH + СН3СООН→СН3СООNa + H2O
Na + + OH- + СН3СООН → СН3СОО- + Na + + H2O
OH- + СН3СООН → СН3СОО- + H2O
Кд>1 Кд>1 Кд>1 Кд<1
2. NaOH + HCl ↔ NaCl + H2O
Na + + OH- + H+ + Cl - ↔ Na + + Cl - + H2O
OH- + H+ ↔ H2O
Вывод: Реакции нейтрализации проходят при участии сильного основания и кислоты. В зависимости от добавленного электролита используется разное кол-во кислоты, участвующей в реакции.
Опыт №4 Условия выпадения осадка.
1. Pb(NO3)2 + 2KCl = PbCl2 + 2KNO3
нитрат свинца хлорид калия хлорид свинца нитрат калия
Pb2+ + 2NO3- + 2K+ + 2Cl- = Pb2+ + 2Cl- + 2K+ + 2NO3-
(Реакция не прошла, осадка не образовалось).
ПК [Pb2+][2Cl-]=6,25*10-8 < ПРPbCl2 = 2,4*10-4, значит осадок не выпадает.
2. Pb(NO3)2 + 2KI = PbI2↓ + 2KNO3
Pb2+ + 2NO3 - + 2K + + 2I- = PbI2↓ + 2K + + 2NO3 -
Pb2+ + 2I- = PbI2↓
(Образовался блестящий осадок ярко-желтого цвета)
ПК [Pb2+][2I-]=6,25*10-6 > ПРPbI2 = 8,7*10-9 , значит осадок выпадает.
Вывод: Условия выпадения осадка является разница между ПК(произведение концентрации) и ПР(произведение растворимости). Если ПК > ПР, то осадок выпадает, если ПК < ПР, то осадка не образуется.
Опыт №5 Влияние величины ПР электролита на его способность к химическому взаимодействию.
1. FeSO4 + Na2S → FeS↓ + Na2SO4
сульфат железа II сульфид натрия сульфид железа II сульфат натрия
Получился осадок черного цвета.
Fe2+ + SO4 -2 + 2Nа + + S2- → FeS↓ + 2Na + + SO4 2-
Fe2+ + S2- → FeS↓
FeS↓ + 2HCl = FeCl2 + H2S
Осадок (FeS) растворился, так как раствор изменил цвет и стал светло-серым, а примерно через несколько минут стал голубоватым (мутным).
FeS↓ + 2H+ + 2Cl - = Fe2+ + 2Cl - + H2S
FeS↓ + 2H+ = Fe2+ + H2S
ПРFeS = 5*10-18 
Кд = КдI * КдII = 5,7*10-8*1,2*10-15 = 6,84*10-23
Реакция протекает в прямом направлении
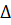 G0 =
G0 = 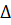 G0298(H2S) +
G0298(H2S) + 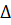 G0298(Fe2+) -
G0298(Fe2+) - 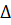 G0298(FeS) - 2
G0298(FeS) - 2 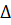 G0298(H+) = -27,97 – 92,26 + +100,88 – 0 = -19,35 кДж
G0298(H+) = -27,97 – 92,26 + +100,88 – 0 = -19,35 кДж
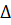 G0 < 0 – реакция протекает самопроизвольно только в прямом направлении.
G0 < 0 – реакция протекает самопроизвольно только в прямом направлении.
2. CuSO4 + Na2S = CuS↓ + Na2SO4
сульфат меди сульфид натрия сульфид меди сульфат натрия
Получится осадок графитного цвета
Cu2+ + SO4 2- + 2Na + + S2- = CuS↓ + 2Na + + SO4 2-
Cu2+ + S2- = CuS↓
CuS↓ + 2HCl = CuCl2 + H2S
Цвет раствора не изменится, никаких признаков протекания реакции нет, реакция не прошла, осадок не образуется.
CuS↓ + 2H+ + 2Cl - = Cu2+ + 2Cl - + H2S
CuS↓ + 2H+ = Cu2+ + H2S
ПРCuS = 3,2*10-38 
КдH2S = 6,84*10-23
Реакция протекает в обратном направлении
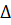 G0298 =
G0298 = 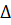 G0298(H2S) +
G0298(H2S) + 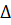 G0298(Cu2+) -
G0298(Cu2+) - 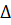 G0298(CuS) - 2
G0298(CuS) - 2 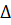 G0298(H+) = -27,97 + 64,98 +
G0298(H+) = -27,97 + 64,98 +
+ 53,76 – 2*0 = 90,77 кДж
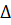 G0 > 0 – реакция протекает самопроизвольно только в обратном направлении (осадок не образуется).
G0 > 0 – реакция протекает самопроизвольно только в обратном направлении (осадок не образуется).
Вывод: В ходе опыта определили влияние ПР на реакционную способность электролита, чем больше ПР, тем способность электролита к химическому взаимодействию лучше, чем меньше величина ПР, тем хуже реакционная способность.






