В химической реакции общего вида nAА+nBB Û nRR+nSS ±Q вещества реагируют в соответствии с соотношением стехиометрической эквивалентности:
 , (1.29)
, (1.29)
где Ni,0 – мольные количества веществ до начала химической реакции, Ni – текущие (конечные) количества, ni - стехиометрические коэффициенты.
Количество всех у чтников и продуктов реакции (Ni,хр) рассчитывают по любому из веществ (NА,0), количество которого известно:
Ni,хр =  ×NА,0 ×ХА; (1.30)
×NА,0 ×ХА; (1.30)
mi,хр = 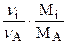 ×mА,0 ×ХА. (1.31)
×mА,0 ×ХА. (1.31)
Здесь m и M – массa и молярная масса веществ соответственно.
Расчет расходной части баланса
На выходе из реактора (расходная часть баланса) поток состоит из продуктов реакции, непрореагировавших исходных веществ (при ХА<1), и веществ, не участвующих в химических превращениях (инертов). Количество каждого компонента в расходной части баланса рассчитывают по формуле:
ni = ni,0 - ni,хр, (1.32)
где ni,хр – количество вещества, израсходованного (для исходных) или образовавшегося (для продуктов) в результате реакции.
Сводная таблица материального баланса
Для реакции типа nAА+nBB Û nRR+nSS, протекающей в присутствии инерта I, результаты расчета материального баланса рекомендуется представлять в табличном виде (табл.1.3):
Таблица 1.3.
Сводная таблица материального баланса
| В-во | Mi, кг/кмоль | ПРИХОД | Реакция | РАСХОД | |||
| ni,0, кмоль/ч | mi,0, кг/ч | ni | ni,х, кмоль/ч | ni, кмоль/ч | mi, кг/ч | ||
| А | МА | NA,0 | mA,0 | nA | NA,0×ХА | nA,0 - NА,хp | NA ×MA |
| B | М B | NB,0 | mB,0 | nB |  NA,0×ХА NA,0×ХА
| nА,0 - NА,хp | NB ×MB |
| R | МR | NR,0 | mR,0 | -nr |  NA,0×ХА NA,0×ХА
| nR,0 - NR,хp | NR ×MR |
| S | МS | NS,0 | mS,0 | -ns |  NA,0×ХА NA,0×ХА
| nS,0 - NS,хp | NS ×MS |
| И | МИ | NИ,0 | MИ,0 | - | - | nИ=NИ,0 | nИ ×MИ |
| ВСЕГО | S ni,0 | Smi,0 | S ni | S mi |
При необходимости состав равновесной смеси можно представить в мольных либо массовых долях:
 (1.33)
(1.33)
 , (1.34)
, (1.34)
где "-" относится к исходным реагентам,"+"- к продуктам, n - стехиометрические коэффициенты; Dn=nR+nS-(nA+nB); D(niMi)=nRMR+nSMS-nAMA-nBMB.
Примеры решения задач
Простейшие упражнения
Пример 1
Сколько граммов соли и воды содержится в 800 г 12% раствора NaNO3?
Решение
Масса растворенной соли составляет 12% от массы раствора
800×12/100= 96 г,
а масса растворителя – 88% от массы раствора: 800×88/100 = 704 г.
Пример 2
Сколько граммов HCl следует растворить в 250 мл г воды для получения 10% раствора HCl?
Решение
250 граммов воды составляют 90% массы раствора, а масса HCl – 10% массы раствора или 250×10/90=27,7 г.
Пример 3
Сколько граммов 10% раствора H2SO4 потребуется для взаимодействия с 100 мл 13,7% раствора Na2CO3 плотностью 1,145 г/см3.
Решение
Масса 100 мл раствора составляет 100×1,145 = 114,5 г. В нем содержится 114,5×0,137 = 15,68 г Na2CO3. Определив молярные массы Na2CO3 и H2SO4, из уравнения реакции Na2CO3+H2SO4=Na2SO4+CO2+H2O по пропорции находим необходимое количество серной кислоты 15,68×98,06/106 = 14,5 г, а 10%-ного раствора H2SO4 потребуется:14,5×100/10 = 145 г.
Пример 4
Сколько мл 32,5% раствора NH3 плотностью 0,888 г/см3требуется для образования сульфата аммония (NH4)2SO4 c 250 мл 27,3% раствора H2SO4 плотностью 1,2 г/см3?
Решение
Масса раствора кислоты составляет 250×1.2 = 300 г.
Раствор содержит 300×0,273 = 81,9 г H2SO4.
По уравнению реакции 2NН3 + H2SO4 = (NН4)2SO4 вычисляем массу аммиака, пошедшего на реакцию:34,06×81,9/98,08 г.
Этому количеству аммиака соответствуют
34,06×81,9×100/98,08×32,5 г или 34,06×81,9×100/98,08×32,5×0,888 = 98,6 мл.
Пример 5
Сколько граммов Na2CO3 прореагирует со 100 мл 4N раствора HCl?
Решение
Вычислим массу HCl в 100 мл 4N раствора:
1000 мл 4N раствора содержат 36,46×4 г HCl
100 мл 4N раствора - х г
х = 36,46×4×0,1 г.
По уравнению реакции Na2CO3 + 2HCl = 2NaCl + H2O + CO2 находим:
106 2×36,46
106 г Na2CO3 - 2×36,46 г HCl
у г Na2CO3 - 0,4×36,46 г HCl
у = 21,2 г.
Пример 6
Вычислить парциальное давление 570 мл газа, собранного над водой при температуре 200С и давлении 104,1 кПа.
Решение
Полное давление складывается из двух величин: парциального давления самого газа и давления насыщенного водяного пара (см. табл.1.2) Следовательно, парциальное давление газа в данном случае равно 104,1 – 2,34=101,76 кПа. Приводя измененный объем газа к нормальным условиям, следует подставить в уравнение не общее давление газовой смеси (104,1 кПа), а парциальное давление газа (101,76 кПа):
 мл.
мл.
Если не учесть поправку на давление паров воды, то вместо найденного объема получим
 мл.
мл.
Ошибка составляет 13 мл, т.е. около 2,5%, что можно допустить только при ориентировочных расчетах.






