Ќещасн≥ випадки (оп≥ки, пораненн€, отруЇнн€) у лаборатор≥њ виникають внасл≥док недостатнього ознайомленн€ працюючих з в≥дпов≥дними ≥нструкц≥€ми з охорони прац≥ ≥ техн≥ки безпеки або в результат≥ необережноњ роботи.
якщо нещасний випадок трапивс€, потерп≥лому треба надати першу допомогу:
1. ѕри попаданн≥ на шк≥ру кислот це м≥сце сл≥д ≥нтенсивно промити водою, а пот≥м 1 %-вим розчином NaHCќ3. ѕри попаданн≥ концентрованоњ сульфатноњ кислоти перед промиванн€м пошкоджену шк≥ру необх≥дно витерти сухим ватним тампоном.
2. ѕри попаданн≥ на шк≥ру розчин≥в луг≥в пошкоджене м≥сце промивають водою, а пот≥м розведеною ацетатною кислотою або насиченим розчином боратноњ кислоти.
3. ѕри попаданн≥ на шк≥ру фенолу, брому ≥ под≥бних њм речовин необх≥дно негайно пошкоджене м≥сце промити в≥дпов≥дни≠ми орган≥чними розчинниками (спиртом, етером тощо).
4. ѕри отруЇнн≥ хлором, бромом, н≥трогену оксидами потерп≥лому необх≥дно дати вдихати пари розведеного розчину амо≠н≥аку ≥ випити молоко.
5. ѕри оп≥ках т≥ла полум'€м необх≥дно негайно промити м≥сце оп≥ку 1 %-вим розчином ћnќ4 ≥ покласти на пошкоджене м≥сце компрес ≥з спиртового розчину тан≥ну.
6. ѕри пор≥зах рану необх≥дно обробити спиртовим розчином йоду ≥ перев'€зати.
7. ѕ≥сл€ наданн€ першоњ допомоги потерп≥лому його терм≥ново необх≥дно в≥дправити до л≥карн≥.
8. ѕри виникненн≥ пожеж≥ в лаборатор≥њ необх≥дно терм≥ново вимкнути вс≥ електричн≥ прилади ≥ перекрити подачу газу. ћ≥сце пожеж≥ необх≥дно засипати п≥ском або накрити протипожежною ковдрою ≥ загасити вогонь за допомогою вогнегасника.
9. «астосовувати воду дл€ гас≥нн€ пожеж≥ треба обережно, тому що вода в де€ких випадках спри€Ї зб≥льшенню пожеж≥.
я ≤—Ќ»… јЌјЋ≤« Ќ≈ќ–√јЌ≤„Ќ»’ —ѕќЋ”
як≥сний анал≥з неорган≥чних сполук дозвол€Ї вста≠новити склад €к ≥ндив≥дуальних неорган≥чних сполук, так ≥ њх су≠м≥шей.
Ѕ≥льш≥сть неорган≥чних сполук Ї електрол≥тами ≥ у водних роз≠чинах знаходитьс€ у вигл€д≥ ≥он≥в. ” зв'€зку з цим €к≥сний анал≥з неорган≥чних сполук под≥л€ють на анал≥з кат≥он≥в ≥ ан≥он≥в.
јЌјЋ≤« ј“≤ќЌ≤¬
≤снуЇ дек≥лька метод≥в систематичного ходу анал≥зу кат≥он≥в: с≥рководневий, амон≥ачно-фосфатний, кислотно-основний тощо. ” с≥рководневому метод≥ застосовуЇтьс€ високотоксичний г≥дроген сульф≥д.
“аблиц€ 5.
ћетоди анал≥тичноњ класиф≥кац≥њ кат≥он≥в
| ћетод анал≥зу | ћетод оснований на |
| —≥рководневий | р≥зн≥й розчинност≥ сульф≥д≥в метал≥в |
| ислотно-основний | р≥зному в≥дношенн≥ кат≥он≥в до кислот (HCl, H2SO4) та основ (NaOH, NH3ЈH2O) |
| јмон≥ачно-фосфатний | р≥зн≥й розчинност≥ фосфат≥в кат≥он≥в у вод≥ та розчин≥ амон≥аку |
ќстанн≥м часом найпоширен≥шим Ї анал≥з кат≥он≥в за кислотно-основний методом, в основ≥ €кого лежить р≥зна розчинн≥сть г≥дроксид≥в ≥ де€ких солей, утворених кат≥онами ≥ сильними кислотами.
” пос≥бнику описано €к≥сний метод анал≥зу кат≥он≥в кислотно-основним методом на приклад≥ ≥он≥в, €к≥ найчаст≥ше вход€ть до складу л≥карських препарат≥в або реагент≥в, що застосовуютьс€ у фармацевтичному анал≥з≥.
|
|
|
“аблиц€ 6.
ласиф≥кац≥€ кат≥он≥в за кислотно-основним методом
| √рупа | ат≥они | √руповий реагент | –озчинн≥сть сполуки |
| ≤ | Na+, K+, NH  , Li+ , Li+
| Ќе маЇ | ’лориди, сульфати ≥ г≥дроксиди розчин€ютьс€ у вод≥. |
| ≤≤ | Ag+, Pb2+, Hg 
| HCl | ’лориди не розчин€ютьс€ у вод≥. |
| ≤≤≤ | Ba2+, Sr2+, Ca2+ | H2SO4 + C2H5OH | —ульфати не розчин€ютьс€ у вод≥. |
| IV | Al3+, Zn2+, Cr3+, As(III), As(V), Sn(II), Sn (IV) | Ќадлишок конц. NaOH, H2O2 | √≥дроксиди не розчин€ютьс€ у вод≥, але розчин€ютьс€ в надлишку розчину лугу. |
| V | Fe2+, Fe3+, Mg2+, Mn2+, Bi3+, Sb(III), Sb(V) | Ќадлишок конц. NH3ЈH2O | √≥дроксиди не розчин€ютьс€ н≥ у вод≥, н≥ в надлишку розчину лугу. |
| VI | Co2+, Ni2+, Cd2+, Cu2+, Hg2+ | Ќадлишок конц. NH3ЈH2O | √≥дроксиди не розчин€ютьс€ у вод≥, надлишку лугу, але розчин€ютьс€ в надлишку амон≥аку. |
ЋјЅќ–ј“ќ–Ќј –ќЅќ“ј
–≈ј ÷≤ѓ ј“≤ќЌ≤¬ ≤ јЌјЋ≤“»„Ќќѓ √–”ѕ» ( +, Na+, NH4+)
ћета: ¬ивчити реакц≥њ кат≥он≥в ≤ анал≥тичноњ групи та умови њх виконанн€.
«агальна характеристика
ƒо першоњ анал≥тичноњ групи кат≥он≥в в≥днос€тьс€ кат≥они лужних метал≥в: кат≥они ал≥ю +, Ќатр≥ю Na+, а також комплексний йон амон≥ю NH4+. ≤он амон≥ю за х≥м≥чними властивост€ми близький до ≥он≥в ал≥ю: њх ≥онн≥ рад≥уси дуже близьк≥. ¬с≥ ц≥ кат≥они утворюють сполуки з ≥онним типом х≥м≥чного звТ€зку ≥ б≥льш≥сть з них добре розчин€Їтьс€ у вод≥. “ому вони не мають загального групового реагенту ≥ тим самим в≥др≥зн€ютьс€ в≥д кат≥он≥в ≥нших анал≥тичних груп.
√≥дратован≥ ≥они ал≥ю, Ќатр≥ю, амон≥ю безбарвн≥. —полуки K+ ≥ Na+ легко утворюють пересичен≥ розчини. “ому дл€ прискоренн€ кристал≥зац≥њ њхн≥х осад≥в необх≥дно терти скл€ною паличкою внутр≥шню ст≥нку проб≥рки, в €к≥й в≥дбуваЇтьс€ реакц≥€.
–еакц≥њ ал≥й-кат≥она
1. ƒ≥€ натр≥й гексан≥трокобальтату(≤≤≤) Na3[Co(Nќ2)6 ] (фармакопейна реакц≥€)
≤они ал≥ю з розчином натр≥й гексан≥трокобальтату(≤≤≤) утворюють жовтий кристал≥чний осад кал≥й-натр≥й гексан≥трокобальтату(≤≤≤).
2KCl + Na3[Co(Nќ2)6] → K2Na[Co(Nќ2)6]↓ + 2NaCl
2K+ + Na+ +[Co(Nќ2)6]3- F K2Na[Co(Nќ2)6]↓
÷ей осад розчин€Їтьс€ в м≥неральних кислотах з утворенн€м нест≥йкоњ кислоти Ќ3[Co(NO2)6] при рЌ<4, €ка розкладаЇтьс€ на HNO2 ≥ Co(NO2)3.
K2Na[Co(Nќ2)6]↓ + 3HCl → 2KCl + NaCl + 3HNO2 + Co(NO2)3
K2Na[Co(Nќ2)6]↓ + 3H+ F 2K+ + Na+ + 3HNO2 + Co3+ + 3NO2-
Ћуги розкладають осад з утворенн€м бурого осаду Co(OH)3.
K2Na[Co(Nќ2)6]↓ + 3NaOH → Co(OH)3 ↓ + 4NaNO2 + 2KNO2
K2Na[Co(Nќ2)6]↓ + 3OH- F Co(OH)3 ↓ + 2 + + Na+ + 6NO2-
¬иконанн€ досл≥ду: до 2-3 крапель розчину сол≥ ал≥ю додати 1-2 крапл≥ розчину Na3[Co(Nќ2)6]. —постер≥гати по€ву жовтого кристал≥чного осаду. ќсад под≥лити на дв≥ проб≥рки. ¬ одну проб≥рку додати 1-2 крапл≥ розчину м≥неральноњ кислоти, в другу 1-2 крапл≥ розчину NaOH. ƒосл≥дити розчинн≥сть осаду K2Na[Co(Nќ2)6] у кислотах та лугах.
2. ƒ≥€ натр≥й г≥дрогентартрату NaHC4H4O6 (фармакопейна реакц≥€)
≤они ал≥ю з розчином натр≥й г≥дрогентартрату утворюють кристал≥чний б≥лий осад кал≥й г≥дрогентартрату. –еакц≥€ прискорюЇтьс€ при охолодженн≥ та потиранн≥ по ст≥нках проб≥рки скл€ною паличкою.
KCl + NaHC4H4O6 → KHC4H4O6↓ + NaCl
K+ + HC4H4O  F KHC4H4O6↓
F KHC4H4O6↓
ќсад розчин€Їтьс€ в м≥неральних кислотах та лугах.
KHC4H4O6↓ + HCl → KCl + H2C4Ќ4O6
KHC4H4O6↓ + H+ F K+ + H2C4Ќ4O6
|
|
|
KHC4H4O6↓ + KOH → K2C4H4O6 + H2O
KHC4H4O6↓ + OH- F C4H4O  + H2O
+ H2O
¬иконанн€ досл≥ду: до 5-6 крапель розчину сол≥ ал≥ю додати 2-3 крапл≥ розчину натр≥й г≥дрогентартрату NaHC4H4O6. –озчин у проб≥рц≥ охолодити п≥д струменем води, потерти скл€ною паличкою по њњ ст≥нках. —постер≥гати по€ву б≥лого кристал≥чного осаду. ќсад под≥лити на дв≥ проб≥рки. ¬ одну проб≥рку додати 1-2 крапл≥ розчину м≥неральноњ кислоти, в другу 1-2 крапл≥ розчину NaOH. ƒосл≥дити розчинн≥сть осаду KHC4H4O6.
3. ƒ≥€ натр≥й тетрафен≥лборатуNa[B(C6H5)4] (фармакопейна реакц≥€)
≤они ал≥ю утворюють з розчином натр≥й тетрафен≥лборату кристал≥чний осад б≥лого кольору.
KCl + Na[B(C6H5)4] → K[B(C6H5)4]↓ + NaCl
K+ + [B(C6H5)4]- F K[B(C6H5)4]↓
¬иконанн€ досл≥ду: до 3-4 крапель розчину сол≥ ал≥ю додати 3-4 крапл≥ розчину натр≥й тетрафен≥лборату Na[B(C6H5)4] ≥ 1 краплю розчину NaOH, щоб уникнути присутност≥ ≥он≥в амон≥ю. —постер≥гати по€ву б≥лого кристал≥чного осаду.
4. –еакц≥€ забарвленн€ полум'€ (реакц≥€ фармакопейна)
—ол≥ ал≥ю забарвлюють безбарвне полумТ€ пальника у ф≥олетовий кол≥р, або при розгл€данн≥ кр≥зь синЇ скло Ц у пурпурово-червоний.
¬иконанн€ досл≥ду: очищеною ≥ розжареною н≥хромовою дротинкою з петлею захопити с≥ль ал≥ю ≥ внести у безбарвну частину полумТ€ пальника. —постер≥гати зм≥ну забарвленн€ полумТ€ у ф≥олетовий кол≥р.
–еакц≥њ Ќатр≥й-кат≥она
1. ƒ≥€ розчину метоксифен≥лацетатноњ кислотиC6H5CH(OCH3)COOH (фармакопейна реакц≥€)
≤они Ќатр≥ю при д≥≥ розчину метоксифен≥лацетатноњ кислоти утворюють обТЇмний б≥лий кристал≥чний осад.
NaCl + 2C6H5CH(OCH3)COOH → C6H5CH(OCH3)COOHЈC6H5CH(OCH3)COONa↓ + HCl
Na+ + 2C6H5CH(OCH3)COOH F C6H5CH(OCH3)COOHЈC6H5CH(OCH3)COONa ↓ + H+
¬ результат≥ реакц≥њ утворюЇтьс€ молекул€рна сполука кислоти та њњ натр≥Ївоњ сол≥.
¬иконанн€ досл≥ду: до 3-4 крапель розчину сол≥ Ќатр≥ю додати 5-6 крапель розчину метоксифен≥лоцтовоњ кислоти. –озчин охолодити п≥д струменем води. —постер≥гати по€ву об'Їмного б≥лого кристал≥чного осаду.
2.ƒ≥€ кал≥й гексаг≥дроксостибату(V) K[Sb(OH)6] (фармакопейна реакц≥€)
онцентрован≥ розчини солей Ќатр≥ю при взаЇмод≥њ з кал≥й гексаг≥дроксостибатом(V) в нейтральному або слабколужному середовищ≥ утворюють б≥лий кристал≥чний осад.
NaCl + K[Sb(OH)6] → Na[Sb(OH)6]↓ + KCl
Na+ + [Sb(OH)6]- F Na[Sb(OH)6]↓
”творенню осаду спри€Ї охолодженн€ розчину ≥ потиранн€ скл€ною паличкою по внутр≥шн≥х ст≥нках проб≥рки.
¬иконанн€ досл≥ду: до 2-3 крапель розчину сол≥ Ќатр≥ю додати 2-3 крапл≥ розчину кал≥й гексаг≥дроксостибату K[Sb(OH)6]. –озчин у проб≥рц≥ охолодити п≥д струменем води, потерти скл€ною паличкою по њњ ст≥нках. —постер≥гати по€ву б≥лого кристал≥чного осаду.
3. ƒ≥€ цинктриуран≥л октаацетату Zn(UO2)3(CH3COO)8.
≤они Ќатр≥ю з цинктриуран≥л октаацеацетатом у нейтральних або ацетатнокислих розчинах утворюють жовтий кристал≥чний осад натр≥йцинктриуран≥л нонаацетат-вода(1:9).
NaCl + Zn(UO2)3(CH3COO)8 + CH3COOH + 9H2O → NaZn(UO2)3(CH3COO)9Ј9H2O↓ + HCl
Na+ + Zn2+ +3UO  + 8CH3COO- + CH3COOH + 9H2O F NaZn(UO2)3(CH3COO)9Ј9H2O↓ + H+
+ 8CH3COO- + CH3COOH + 9H2O F NaZn(UO2)3(CH3COO)9Ј9H2O↓ + H+
ѕ≥д м≥кроскопом кристали NaZn(UO2)3(CH3COO)9Ј9H2O мають вигл€д правильних октаедр≥в або тетраедр≥в.
¬иконанн€ досл≥ду: до 2-3 крапель розчину сол≥ Ќатр≥ю додати 2-3 крапл≥ розчину цинктриуран≥л октаацетату Zn(UO2)3(CH3COO)8 та 2-3 крапл≥ ацетатноњ кислоти. —постер≥гати по€ву жовтого кристал≥чного осаду. ќсад роздивитис€ п≥д м≥кроскопом.
4. –еакц≥€ забарвленн€ полумТ€ (реакц≥€ фармакопейна)
—ол≥ Ќатр≥ю забарвлюють безбарвне полумТ€ пальника в жовтий кол≥р.
¬иконанн€ досл≥ду: очищену ≥ розжарену н≥хромову дротинку змочити хлоридною кислотою, захопити с≥ль Ќатр≥ю ≥ внести у безбарвну частину полумТ€ пальника. —постер≥гати зм≥ну забарвленн€ полумТ€ в жовтий кол≥р.
–еакц≥њ амон≥й-кат≥она
1. ƒ≥€ луг≥в (фармакопейна реакц≥€)
—ол≥ амон≥ю реагують з розчинами NaOH ≥ KOH при нагр≥ванн≥ з вид≥ленн€м амон≥аку.
|
|
|
NH4Cl + NaOH  NH3ЈH2O + NaCl
NH3ЈH2O + NaCl
NH  + OH-
+ OH-  NH3ЈH2O
NH3ЈH2O
NH3ЈH2O  NH3↑ + H2O
NH3↑ + H2O
јмон≥ак можна визначити за запахом або за забарвленн€м вологого червоного лакмусового пап≥рц€ в син≥й кол≥р. –еакц≥€ чутлива ≥ специф≥чна, тому визначенню ≥он≥в амон≥ю не заважають ≥нш≥ кат≥они.
¬иконанн€ досл≥ду: до 3-4 крапель розчину сол≥ амон≥ю додати 2-3 крапл≥ розчину лугу. –озчин у проб≥рц≥ нагр≥ти. ƒо отвору проб≥рки п≥днести вологий червоний лакмусовий пап≥рець. —постер≥гати зм≥ну кольору пап≥рц€.
2. ƒ≥€ реактиву Ќесслера (дикал≥й тетрайодомеркурат(≤≤) у сум≥ш≥ з кал≥й г≥дроксидом) K2[HgI4] + KOH.
≤они амон≥ю з реактивом Ќесслера утворюють червоно-бурий або жовто-бурий (при невеликих концентрац≥€х ≥ону амон≥ю) аморфний осад ам≥дного комплексу меркур≥ю(≤≤).
NH4Cl + 2K2[HgI4] + 4KOH → [OHg2NH2]I↓ + 7KI + KCl + 3H2O
NH  +4K+ +2[HgI4]2- +4K+ +4OH- F [OHg2NH2]I↓ +7K+ +7I- +K+ +Cl- +3H2O
+4K+ +2[HgI4]2- +4K+ +4OH- F [OHg2NH2]I↓ +7K+ +7I- +K+ +Cl- +3H2O
NH  + 2[HgI4]2- + 4OH- F [OHg2NH2]I↓ + 7I- + 3H2O
+ 2[HgI4]2- + 4OH- F [OHg2NH2]I↓ + 7I- + 3H2O
ѕри малих концентрац≥€х ≥он≥в амон≥ю осад не утворюЇтьс€, але розчин забарвлюЇтьс€ в жовтий кол≥р. –еакц≥ю необх≥дно проводити в нейтральному або лужному середовищ≥, в кислому середовищ≥ реагент руйнуЇтьс€ з утворенн€м червоного осаду Hg≤2. ÷€ реакц≥€ надзвичайно чутлива.
¬иконанн€ досл≥ду: до 3-4 крапель розчину сол≥ амон≥ю додати 1-2 крапл≥ реактиву Ќесслера. —постер≥гати по€ву жовто- бурого осаду.
3. ƒ≥€ магн≥й оксиду MgO (фармакопейна реакц≥€)
ƒо досл≥дного розчину додають магн≥й оксид, €кий реагуЇ з водою з утворюванн€м магн≥й г≥дроксиду:
MgO + H2O → Mg(OH)2 → Mg2+ + 2OH-
Mg(OH)2 вид≥л€Ї амон≥ак ≥з солей так само, €к луги, але не розкладаЇ летк≥ основи. р≥зь розчин продувають пов≥тр€, ≥ газ NH3, що вид≥л€Їтьс€, поглинають розчином хлоридноњ кислоти. ѕри цьому утворюютьс€ ≥они амон≥ю:
NH3↑ + HCl → NH4Cl
NH3↑ + H+ F NH4+
ƒл€ доведенн€ присутност≥ ≥он≥в амон≥ю, до розчину, що поглинаЇ амон≥ак, додають розчин Na3[Co(Nќ2)6]. ”творюЇтьс€ жовтий осад (NH4)2Na[Co(Nќ2)6].
2NH4+ + Na+ +[Co(Nќ2)6]3- F (NH4)2Na[Co(Nќ2)6]↓
¬иконанн€ досл≥ду: до 2-3 см3 досл≥джуваного розчину додати 0,2 г магн≥й оксиду MgO. р≥зь розчин попустити струм≥нь пов≥тр€. √аз, що вид≥ливс€, спр€мувати в проб≥рку з сум≥шшю 1 см3 0,1 ћ розчину хлоридноњ кислоти HCl та 1-2 крапель розчину ≥ндикатору метилового червоного. —постер≥гати зм≥ну забарвленн€ розчину. ƒодати у розчин 2-3 крапл≥ св≥жоприготованого розчину Na3[Co(Nќ2)6]. —постер≥гати по€ву жовтого осаду.
онтрольн≥ питанн€
1. ”каж≥ть кат≥они ≤ анал≥тичноњ групи.
2. ¬каж≥ть, чому кат≥они ≤ анал≥тичноњ групи не мають групового реагенту.
3. ¬каж≥ть умови ви€вленн€ ≥он≥в ал≥ю з натр≥й г≥дрогентартратом.
4. ¬каж≥ть умови ви€вленн€ ≥он≥в ал≥ю з реактивом натр≥й гексан≥трокобальтатом(≤≤≤).
5. ¬каж≥ть умови ви€вленн€ ≥он≥в Ќатр≥ю з розчином кал≥й гексаг≥дростибату(V).
6. ѕо€сн≥ть, чому реакц≥ю кат≥он≥в амон≥ю з реактивом Ќесслера потр≥бно проводити т≥льки в лужному середовищ≥.
7. ѕо€сн≥ть, чому ви€вленн€ ≥он≥в амон≥ю д≥Їю лугу Ї специф≥чною реакц≥Їю.
Ѕ≥олог≥чна роль та практичне використанн€ кат≥он≥в ≤ анал≥тичноњ групи
та њх сполук в медицин≥ та фармац≥њ
Ќатр≥й Ї основним позакл≥тинним ≥оном. ¬≥н знаходитьс€ в плазм≥ кров≥, л≥мф≥, травних соках. «агальна масова частка Ќатр≥ю в орган≥зм≥ людини складаЇ 0,25%. ƒобова потреба орган≥зму людини в натр≥њ дор≥внюЇ 4-7 г. Ќатр≥й граЇ в орган≥зм≥ людини важливу б≥олог≥чну роль. ≤он Ќатр≥ю бере участь в забезпеченн≥ осмотичного тиску в кл≥тинах (осмотичний тиск на 60% зумовлений сол€ми натр≥ю), п≥дтримуЇ кислотно-основну р≥вновагу (рЌ) в орган≥зм≥. ѕри зм≥нюванн≥ к≥лькост≥ ≥он≥в Ќатр≥ю в орган≥зм≥ в≥дбуваютьс€ порушенн€ функц≥й нервовоњ, серцево-судинноњ системи, гладких та скелетних м'€з≥в. Ќатр≥й впливаЇ на роботу фермент≥в ≥ бере участь в регул€ц≥њ водного обм≥ну.
|
|
|
Ѕагато сполук Ќатр≥ю знаход€ть використанн€ в медицин≥ та фармац≥њ. Ќатр≥й хлорид NaCl у вигл€д≥ ≥зотон≥чного (0,9%) розчину призначаЇтьс€ внутр≥шньовенно, п≥дшк≥рно, ректально при отруЇнн€х, токсичн≥й диспепс≥њ, холер≥, сильному блюванн≥, великих кровозбитках, тощо; зовн≥шньо Ц дл€ промиванн€ ран слизовоњ оболонки очей, носа. –озчин ц≥Їњ сол≥ призначаЇтьс€ внутр≥шньовенно при легеневих, шлункових та ≥нших внутр≥шн≥х кровотечах, його застосовують дл€ промиванн€ шлунка, при отруЇнн≥ аргентум(≤) н≥тратом. «овн≥шньо його застосовують у вигл€д≥ компрес≥в та примочок дл€ л≥куванн€ гн≥йних ран. Ќатр≥й бром≥д NaBr та кал≥й бром≥д KBr використовують €к зас≥б, €кий впливаЇ на нервову систему, регулюЇ њњ д≥€льн≥сть.
Ќатр≥й сульфат Na2SO4 (глауберова с≥ль) призначаЇтьс€ €к проносне, при отруЇнн€х л≥кувальними засобами (розчинними сол€ми Ѕар≥ю та ѕлюмбуму, з €кими Na2SO4 даЇ нерозчинн≥ сполуки BaSќ4, PbSќ4), харчовими продуктами.
Ќатр≥й г≥дрогенкарбонат NaHCO3 нейтрал≥зуЇ кислотн≥сть шлункового вм≥сту, п≥двищуЇ лужн≥ резерви кров≥ та зн≥маЇ €вища ацидозу. ”середину призначаЇтьс€ при г≥перацидному гастрит≥, виразц≥ шлунка та дванадц€типалоњ кишки. «овн≥шньо Ц дл€ промиванн€ та полосканн€ слизовоњ оболонки очей, ротовоњ порожнини, дл€ ≥нгал€ц≥й, нейтрал≥зац≥њ кислот, €к≥ попали на слизов≥ оболонки та шк≥ру.
Ќатр≥й г≥дроксид NaOH у вигл€д≥ 10% водного розчину входить до складу силам≥ну, €кий використовуЇтьс€ в ортопедичн≥й практиц≥. –ад≥оактивний нукл≥д натр≥ю 24Na знайшов широке використанн€ у кл≥н≥чн≥й практиц≥. Ќаприклад, за допомогою м≥ченого Ќатр≥ю вивчаЇтьс€ швидк≥сть кровотоку, в≥н використовуЇтьс€ дл€ л≥куванн€ де€ких форм лейкем≥њ.
Ќатр≥й тетраборат Na2B4O7Ј10Ќ2ќ Ц використовують зовн≥шньо €к протим≥кробний та протизапальний зас≥б.
ал≥й входить до складу орган≥в ≥ тканин орган≥зму. …ого топограф≥€: печ≥нка, нирки, серце, мозок, кров. «агальна масова частка кал≥ю в орган≥зм≥ складаЇ 0,22%. ал≥й Ї основним внутр≥шньокл≥тинним ≥оном.
¬нутр≥шньокл≥тинна концентрац≥€ ≥он≥в ал≥ю значно вища, н≥ж ≥он≥в Ќатр≥ю. ÷е пов'€зано з тим, що мембрани кл≥тин б≥льш прониклив≥ дл€ ≥он≥в ал≥ю. ¬ орган≥зм≥ пост≥йно ≥снуЇ певне сп≥вв≥дношенн€ ≥он≥в ал≥ю ≥ Ќатр≥ю. «авд€ки цьому сп≥вв≥дношенню п≥дтримуЇтьс€ нормальний ритм м'€зовоњ роботи (м'€зи серц€). ≤они ал≥ю разом з ≥онами Ќатр≥ю створюють систему, €ка забезпечуЇ ≥зотон≥чн≥сть кл≥тин ≥ навколишнього середовища. ≤они ал≥ю беруть участь у синтез≥ б≥лк≥в, вуглевод≥в, вход€ть до складу де€ких фермент≥в та впливають на њх активн≥сть.
—ол≥ ал≥ю швидко всмоктуютьс€ при введенн≥ усередину та швидко вивод€тьс€ з орган≥зму нирками. ¬они не можуть бути зам≥нен≥ в орган≥зм≥ людини н≥€кими ≥ншими сол€ми.
јмон≥й. ¬ орган≥зм≥ Ќ≥троген знаходитьс€ в ступен≥ окисненн€ -3, вход€чи до складу самих р≥зноман≥тних сполук: ам≥нокислот, ам≥докислот, в≥там≥н≥в (первинн≥ ам≥ни), пол≥пептид≥в (вторинн≥ ам≥ни).
јмон≥й хлорид NH4Cl використовуЇтьс€ €к сечог≥нний зас≥б при набр€ках, зумовлених серцево-судинною недостатн≥стю, дл€ посиленн€ д≥њ ртутних сечог≥нних препарат≥в. јмон≥й хлорид використовують €к в≥дхаркуючий зас≥б.
Ўироко використовуЇтьс€ в медичн≥й практиц≥ нашатирний спирт медичний (9,5-10,5% амон≥аку). ѕри прийманн≥ усередину або при вдиханн≥ пар≥в нашатирний спирт подразнюЇ рецептори верхн≥х дихальних шл€х≥в. ¬икористовуЇтьс€ при непритомност≥, зовн≥шньо Ц в х≥рург≥њ дл€ митт€ рук €к мийний та дезинф≥куючий зас≥б.
ЋјЅќ–ј“ќ–Ќј –ќЅќ“ј
–≈ј ÷≤ѓ ј“≤ќЌ≤¬ II јЌјЋ≤“»„Ќќѓ √–”ѕ» (Ag+, Hg  , Pb2+)
, Pb2+)
ћета: ¬ивчити реакц≥њ кат≥он≥в ≤≤ анал≥тичноњ групи та умови њх виконанн€.
«агальна характеристика
ƒо II анал≥тичноњ групи в≥днос€тьс€ кат≥они р-елемента –b2+ ≥ d-елемент≥в Ag+ ≥ Hg  .
.
ат≥они II анал≥тичноњ групи утворюють нерозчинн≥ галоген≥ди (кр≥м аргентум(≤) фториду), сульфати, сульф≥ди, хромати, фосфати, арсен≥ти, арсенати, г≥дроксиди та карбонати, що по€снюЇтьс€ високою пол€ризац≥йною д≥Їю цих кат≥он≥в.
√руповим реагентом на II анал≥тичну групу Ї розчин 2ћ хлоридноњ кислоти HCl, €кий дозвол€Ї селективно в≥докремити ц≥ кат≥они у вигл€д≥ осад≥в в≥дпов≥дних хлорид≥в. ат≥они ≤, IIIЦVI анал≥тичних груп при цьому залишаютьс€ в розчин≥. ƒл€ кат≥он≥в II анал≥тичноњ групи характерн≥ реакц≥њ комплексоутворенн€, а дл€ Hg  Ц реакц≥њ окисненн€-в≥дновленн€.
Ц реакц≥њ окисненн€-в≥дновленн€.
—ол≥ кат≥он≥в ≤≤ анал≥тичноњ групи здеб≥льшого безбарвн≥, але ≥нколи вони мають кол≥р, €кщо до њх складу вход€ть забарвлен≥ ан≥они.
«агальноанал≥тичн≥ реакц≥њ кат≥он≥в II анал≥тичноњ групи
|
|
|
1. ƒ≥€ 2 ћ розчину хлоридноњ кислотиHCl (д≥€ групового реагенту)
ат≥они II анал≥тичноњ групи утворюють з хлоридною кислотою б≥л≥ осади.
AgNO3 + Ќ—l → AgCl↓ + ЌNO3
Ag+ + Cl- F AgCl↓  = 1,78Ј10-10
= 1,78Ј10-10
Hg2(NO3)2 + 2ЌCl → Hg2Cl2↓ + 2ЌNO3
Hg  + 2Cl- F Hg2Cl2↓
+ 2Cl- F Hg2Cl2↓  = 1,3Ј10-18
= 1,3Ј10-18
Pb(NO3)2 + 2HCl → PbCl2↓ + 2ЌNO3
Pb2+ + 2Cl- F PbCl2↓  = 1,6Ј10-5
= 1,6Ј10-5
ќсад плюмбум(≤≤) хлорид PbCl2 маЇ найб≥льшу розчинн≥сть серед хлорид≥в кат≥он≥в II анал≥тичноњ групи: в≥н повн≥стю розчин€Їтьс€ в гар€ч≥й вод≥.
¬иконанн€ досл≥ду: в окрем≥ проб≥рки до 2-3 крапель розчин≥в кат≥он≥в II анал≥тичноњ групи додати по 2-3 крапл≥ розчину 2 ћ хлоридноњ кислоти ЌCl. —постер≥гати по€ву осад≥в та зазначити њх кол≥р.
2. ƒ≥€ луг≥в
ат≥они Ag+ ≥ Pb2+ утворюють з розчинами NaOH ≥ ќЌ б≥л≥ осади. јргентум(≤) г≥дроксид швидко бур≥Ї внасл≥док перетворенн€ його в Ag2ќ.
AgNO3 + NaOH → AgOH↓ + NaNO3
Ag+ + OH- F AgOH↓
2AgOH↓ → Ag2O↓ + H2O
ѕлюмбум(≤≤) г≥дроксид маЇ амфотерн≥ властивост≥ ≥ розчин€Їтьс€ в надлишку реагенту:
Pb(NO3)2 + 2NaOH → Pb(OH)2↓ + 2NaNO3
Pb2+ + 2OH- F Pb(OH)2↓
Pb(OH)2↓ + 2NaOH → Na2[Pb(OH)4]
Pb(OH)2↓ + 2OH- F [Pb(OH)4]2-
ƒимеркур≥й(≤)-кат≥они при взаЇмод≥њ з лугами утворюють чорний осад димеркур≥й(≤) оксиду:
Hg2(NO3)2 + 2NaOH Ѓ Hg2O↓ + H2O + 2NaNO3
Hg  + 2OH- F Hg2O↓ + H2O
+ 2OH- F Hg2O↓ + H2O
¬иконанн€ досл≥ду: в окрем≥ проб≥рки до 2-3 крапель розчин≥в кат≥он≥в ≤≤ анал≥тичноњ групи додати по 2-3 крапл≥ розчину лугу NaOH або ќЌ. —постер≥гати по€ву осад≥в та зазначити њх кол≥р.
3. ƒ≥€ розчину кал≥й йодиду ≤
ат≥они ≤≤ анал≥тичноњ групи утворюють з йодид-≥онами забарвлен≥ малорозчинн≥ сполуки:
AgNO3 + KI → AgI↓ + KNO3
Ag+ + I- F AgI↓ жовтий осад
Hg2(NO3)2 + 2KI → Hg2I2↓ + 2KNO3
Hg  + 2I- F Hg2I2↓ зелений осад
+ 2I- F Hg2I2↓ зелений осад
Pb(NO3)2 + 2KI → PbI2↓ + 2KNO3
Pb2+ + 2I- F PbI2↓ золотисто-жовтий осад
¬иконанн€ досл≥ду: в окрем≥ проб≥рки до 2-3 крапель розчин≥в кат≥он≥в ≤≤ анал≥тичноњ групи додати по 2-3 крапл≥ розчину кал≥й йодиду ≤. —постер≥гати по€ву осад≥в та зазначити њх кол≥р.
4. ƒ≥€ розчину кал≥й хромату K2CrO4
ат≥они ≤≤ анал≥тичноњ групи утворюють з хромат-≥онами забарвлен≥ осади:
2AgNO3 + K2CrO4 → Ag2CrO4↓ + 2KNO3
2Ag+ + CrO  F Ag2CrO4¯ цегл€но-червоний осад
F Ag2CrO4¯ цегл€но-червоний осад
Hg2(NO3)2 + K2CrO4 → Hg2CrO4↓ +2KNO3
Hg  + CrO
+ CrO  F Hg2CrO4↓ червоний осад
F Hg2CrO4↓ червоний осад
Pb(NO3)2 + K2CrO4 → PbCrO4↓ + 2KNO3
Pb2+ + CrO  F PbCrO4↓ жовтий осад
F PbCrO4↓ жовтий осад
¬иконанн€ досл≥ду: в окрем≥ проб≥рки до 2-3 крапель розчин≥в кат≥он≥в ≤≤ анал≥тичноњ групи додати по 2-3 крапл≥ розчину кал≥й хромату K2CrO4. —постер≥гати по€ву осад≥в та зазначити њх кол≥р.
–еакц≥€ јргентум(≤)-кат≥она
–еакц≥€ в≥дкритт€ јргентум(≤)-кат≥он≥в в≥дбуваЇтьс€ у три стад≥њ:
≤.ƒ≥€ 2ћ розчину хлоридноњ кислоти HCl: утворюЇтьс€ б≥лий аморфний осад аргентум(≤) хлориду:
AgNO3 + HCl →AgCl↓ + HNO3
Ag+ + Cl- F AgCl↓
≤≤. ƒ≥€ розчину амон≥аку NH3ЈH2O: утворений осад аргентум(≤) хлориду розчин€Їтьс€ з утворенн€м комплексноњ сполуки диам≥наргентум(≤) хлорид:
AgCl↓ + 2NH3ЈH2O → [Ag(NH3)2]Cl + 2H2O
AgCl↓ + 2NH3ЈH2O F [Ag(NH3)2]+ + Cl- + 2H2O
≤≤≤. ƒ≥€ розчину н≥тратноњ кислоти HNO3: комплекс [Ag(NH3)2]Cl руйнуЇтьс€ з утворенн€м б≥лого осаду аргентум(≤) хлориду:
[Ag(NH3)2]Cl + 2HNO3 → AgCl↓ + 2NH4NO3
[Ag(NH3)2]+ + Cl- + 2H+ F AgCl↓ + 2NH 
¬иконанн€ досл≥ду: у проб≥рку до 1-2 крапель розчину AgNO3 додати 1-2 крапл≥ 2 ћ розчину хлоридноњ кислоти HCl. —постер≥гати по€ву осаду б≥лого кольору. ѕот≥м додати 2-3 крапл≥ концентрованого розчину амон≥аку NH3ЈH2O. —постер≥гати розчиненн€ осаду. ¬ отриманий розчин додати 4-5 крапель н≥тратноњ кислоти HNO3. —постер≥гати по€ву б≥лого осаду AgCl.
–еакц≥њ ѕлюмбум(≤≤)-кат≥она
1. ƒ≥€ розчину кал≥й йодидуKI
ѕлюмбум(≤≤)-кат≥они при д≥њ розчину кал≥й йодиду KI утворюють осад золотисто-жовтого кольору PbI2.
Pb(NO3)2 + 2KI → PbI2↓ + 2KNO3
Pb2+ + 2I- F PbI2↓
ќсад PbI2 розчин€Їтьс€ в надлишку реагенту з утворенн€м комплексноњ сполуки кал≥й тетрайодоплюмбату(≤≤), а також розчин€Їтьс€ в гар€ч≥й вод≥ та в ацетатн≥й кислот≥.
PbI2↓ + 2KI → K2[PbI4]
PbI2↓ + 2I- F [PbI4]2-
¬иконанн€ досл≥ду: до 1-2 крапель розчину сол≥ ѕлюмбум(≤≤)-кат≥он≥в додати 2-3 крапл≥ розчину кал≥й йодиду KI. —постер≥гати по€ву осаду жовтого кольору. ѕот≥м додати ще 3-5 крапель реагенту. —постер≥гати розчиненн€ осаду.
–еакц≥њ димеркур≥й(≤)-кат≥она
1. ƒ≥€ розчину кал≥й йодиду ≤
ƒимеркур≥й(≤)-кат≥они при д≥њ розчину кал≥й йодиду KI утворюють осад зеленого кольору Hg2I2:
Hg2(NO3)2 + 2KI → Hg2I2↓ + 2KNO3
Hg  + 2I- F Hg2I2↓
+ 2I- F Hg2I2↓
ѕри д≥њ надлишку реагенту осад Hg2I2 розчин€Їтьс€ з утворенн€м дикал≥й тетрайодомеркурату(≤≤) та осаду метал≥чноњ ртут≥.
Hg2I2↓ + 2KI → K2[HgI4] + Hg↓
Hg2I2↓ + 2I- F [HgI4]2- + Hg↓
¬иконанн€ досл≥ду: до 1-2 крапель розчину сол≥ димеркур≥й(≤)-кат≥он≥в додати 2-3 крапл≥ розчину кал≥й йодиду KI. —постер≥гати по€ву осаду зеленого кольору. ѕот≥м додати ще 3-5 крапель реагенту. —постер≥гати розчиненн€ зеленого осаду та утворюванн€ чорного осаду метал≥чноњ ртут≥.
онтрольн≥ питанн€
1. Ќазв≥ть груповий реагент ≤≤ анал≥тичноњ групи. як≥ осади утворюЇ цей реагент з кат≥онами Hg  , Pb2+, Ag+? Ќапиш≥ть р≥вн€нн€ цих реакц≥й.
, Pb2+, Ag+? Ќапиш≥ть р≥вн€нн€ цих реакц≥й.
2. як можливо в≥докремити плюмбум(≤≤) хлорид в≥д ≥нших хлорид≥в кат≥он≥в ≤≤ групи в ход≥ анал≥зу сум≥ш≥?
3. ƒ≥Їю €ких реагент≥в можна п≥дтвердити амфотерн≥ властивост≥ плюмбум(≤≤) г≥дроксиду? Ќапиш≥ть р≥вн€нн€ в≥дпов≥дних реакц≥й.
4. « €кими реагентами кат≥они ≤≤ анал≥тичноњ групи утворюють кольоров≥ осади?
5. Ќапиш≥ть трьохстад≥йну реакц≥ю ви€вленн€ кат≥она Ag+.
Ѕ≥олог≥чна роль та практичне використанн€ кат≥он≥в II анал≥тичноњ групи
та њх сполук у медицин≥ та фармац≥њ
јргентум. «агальна масова частка ≥он≥в јргентуму в орган≥зм≥ людини складаЇ 1Ј10-6 %. Ќакопичуютьс€ вони в печ≥нц≥, нирках, к≥стков≥й тканин≥, залозах внутр≥шньоњ секрец≥њ. ‘≥з≥олог≥чна та б≥олог≥чна роль ≥он≥в јргентуму мало вивчена. ¬ залежност≥ в≥д концентрац≥њ ≥они јргентуму справл€ють в'€жучий, прип≥каючий, протизапальний та бактерицидний вплив.
≤з сполук јргентуму в медицин≥ використовуЇтьс€ аргентум(≤) н≥трат AgNO3 (л€п≥с) - це фармакопейний препарат (антисептичний, прип≥каючий зас≥б), його використовують дл€ дез≥нфекц≥њ слизових оболонок очей, в уролог≥њ, дерматолог≥њ, а також €к реактив при анал≥з≥ багатьох л≥кувальних препарат≥в. —таб≥л≥зован≥ спец≥альними добавками колоњдн≥ розчини јргентуму (альбум≥нати јргентуму) Ц протаргол (комплексна сполука б≥лка з ≥онами јргентуму), коларгол (колоњдне ср≥бло), та ≥нш≥ використовуютьс€ при промиванн≥ сечовив≥дних шл€х≥в; при обробц≥ слизових оболонок верхн≥х дихальних шл€х≥в, в очн≥й практиц≥, х≥рург≥њ та дерматолог≥њ €к в'€жуч≥, антисептичн≥ засоби.
ѕри концентрац≥њ вище 2Ј10-11 моль/дм3 ≥они јргентуму дають бактерицидний ефект. Ќа цьому засновано використанн€ у медицин≥ поср≥блених перев'€зних матер≥ал≥в. Ѕактерицидний пап≥р Ђср≥бн≥ї марл€ ≥ вата Ц це пап≥р, марл€ та вата, змочен≥ розчинами аргентум н≥трату та хлориду. “ак≥ матер≥али, змочен≥ водою, накладають на вражен≥ д≥л€нки при оп≥ках II ступен€, саднах, невеликих ранах ≥ т.д. ѕерев'€зний матер≥ал, покритий найдр≥бн≥шими частинками металевого ср≥бла, використовуЇтьс€ дл€ л≥куванн€ р≥зних шк≥рних захворювань. Ѕактерицидн≥ властивост≥ ср≥бла використовуютьс€ також дл€ знезаражуванн€ та консервуванн€ питноњ води на корабл€х, в польових умовах, а також у плавальних басейнах ≥ т.д.
јргентум(≤) хлорид AgCl Ї складовою частиною фосфатних бактерицидних цемент≥в (пломбуючий матер≥ал). —р≥бло застосовуЇтьс€ в рентген≥ та спектрограф≥њ, а також при виготовленн≥ електронноњ та д≥агностичноњ апаратури.
ѕлюмбум. «агальна масова дол€ ≥он≥в ѕлюмбуму в орган≥зм≥ людини складаЇ 1Ј10-6 %. Ѕ≥олог≥чна роль вивчена мало.
—винець та його сполуки дуже токсичн≥ та викликають хрон≥чн≥ отруЇнн€. ” медицин≥ знаход€ть застосуванн€ так≥ сполуки, €к плюмбум(≤≤) оксид PbO, плюмбум(≤≤) ацетат Pb(CH3COO)2 (фармакопейн≥ препарати), свинцева вода (2% розчин основного плюмбум(≤≤) ацетату [(—Ќ3—ќќ)(ќЌ)–b]), пластир свинцевий (PbO). ѓх застосовують в дерматолог≥њ дл€ примочок та спринцювань €к в'€жучий, протизапальний та протим≥кробний зас≥б.
ћеркур≥й (≤). ≤з сполук ћеркур≥ю (≤) в медицин≥ та фармаколог≥њ використовуЇтьс€ димеркур≥й(≤) дихлорид Hg2Cl2 (каломель). ÷€ сполука застосовуЇтьс€ €к сечог≥нний, проносний зас≥б та при захворюванн€х очей.
ЋјЅќ–ј“ќ–Ќј –ќЅќ“ј
–≈ј ÷≤ѓ ј“≤ќЌ≤¬ III јЌјЋ≤“»„Ќќѓ √–”ѕ» (Ba2+, Ca2+, Sr2+)
ћета роботи: ¬ивчити реакц≥њ кат≥он≥в ≤≤≤ анал≥тичноњ групи та умови њх виконанн€.
«агальна характеристика
ƒо ≤≤≤ анал≥тичноњ групи в≥днос€тьс€ кат≥они лужноземельних метал≥в Ѕар≥ю Ba2+, альц≥ю Ca2+, —тронц≥ю Sr2+, €к≥ належать до головноњ п≥дгрупи другоњ групи пер≥одичноњ системи ƒ.≤.ћенделЇЇва. ƒосить висока пол€ризуюча д≥€ кат≥он≥в III анал≥тичноњ групи призводить до того, що б≥льш≥сть солей цих кат≥он≥в Ц сульфати, карбонати, хромати, оксалати, фосфати практично нерозчинн≥ у вод≥.
√руповим реагентом на ≤≤≤ анал≥тичну групу Ї розчин 1ћ H2SO4 у присутност≥ етилового спирту, €кий впливаЇ на повноту осадженн€ кат≥он≥в ц≥Їњ групи у вигл€д≥ сульфат≥в.
ƒл€ кат≥он≥в ≤≤≤ анал≥тичноњ групи не характерн≥ окисно-в≥дновн≥ реакц≥њ, тому що вони мають пост≥йний ступень окисненн€.
Ѕ≥льш≥сть сполук, що м≥ст€ть кат≥они ц≥Їњ анал≥тичноњ групи, б≥лого кольору, а њх розчини безбарвн≥.
«агальноанал≥тичн≥ реакц≥њ кат≥он≥в III групи
1. ƒ≥€ 1ћ розчину сульфатноњ кислоти H2SO4(д≥€ групового реагенту)
ат≥они ≤≤≤ анал≥тичноњ групи при д≥њ розчину сульфатноњ кислоти утворюють б≥л≥ кристал≥чн≥ осади сульфат≥в.
BaCl2 + H2SO4  BaSO4↓ + 2HCl
BaSO4↓ + 2HCl  = 1,1Ј10-10
= 1,1Ј10-10
Ba2+ + SO  F BaSO4↓
F BaSO4↓
CaCl2 + H2SO4  CaSO4↓ + 2HCl
CaSO4↓ + 2HCl  = 2,5Ј10-5
= 2,5Ј10-5
—а2+ + SO  F CaSO4↓
F CaSO4↓
Sr(NO3)2 + H2SO4  SrSO4↓ + 2HNO3
SrSO4↓ + 2HNO3  = 3,2Ј10-7
= 3,2Ј10-7
Sr2+ + SO  F SrSO4↓
F SrSO4↓
–озчинн≥сть осаду CaSO4 досить велика, тому повного осадженн€ ≥он≥в —а2+ не в≥дбуваЇтьс€. ƒл€ зниженн€ розчинност≥ CaSO4 в розчин треба додати етиловий спирт. ÷е призводить до зниженн€ розчинност≥ осад≥в ус≥х кат≥он≥в III анал≥тичноњ групи. —ульфати ¬а2+, Ca2+, Sr2+ не розчин€ютьс€ у кислотах ≥ лугах. CaSO4 розчин€Їтьс€ у концентрованих розчинах (NH4)2SO4 внасл≥док реакц≥њ комплексоутворенн€.
CaSO4¯ + (NH4)2SO4 → (NH4)2[Ca(SO4)2]
CaSO4↓ + SO  F [Ca(SO4)2]
F [Ca(SO4)2] 
÷ю реакц≥ю ≥нод≥ використовують дл€ вид≥ленн€ ≥он≥в альц≥ю в≥д ≥нших кат≥он≥в ≤≤≤ анал≥тичноњ групи.
¬иконанн€ досл≥ду: в окрем≥ проб≥рки до 2-3 крапель розчин≥в кат≥он≥в ≤≤≤ анал≥тичноњ групи додати по 1-2 крапл≥ 1ћ розчину сульфатноњ кислоти H2SO4 ≥ 1-2 крапл≥ етанолу. —постер≥гати по€ву осад≥в та визначити њх кол≥р.
2. ƒ≥€ карбонат≥в
ат≥они ≤≤≤ анал≥тичноњ групи утворюють з розчинами Na2CO3 ≥ 2CO3 б≥л≥ кристал≥чн≥ осади.
BaCl2 + Na2CO3 → BaCO3↓ + 2NaCl  = 4,0Ј10-10
= 4,0Ј10-10
Ba2+ + CO  F BaCO3↓
F BaCO3↓
CaCl2 + Na2CO3 → CaCO3↓ + 2HCl  = 3,7Ј10-9
= 3,7Ј10-9
—a2+ + CO  F CaCO3↓
F CaCO3↓
Sr(NO3)2 + Na2CO3 → SrCO3↓ + 2NaNO3  = 1,0Ј10-10
= 1,0Ј10-10
Sr2+ + CO  F SrCO3↓
F SrCO3↓
¬иконанн€ досл≥ду: в окрем≥ проб≥рки до 2-3 крапель розчин≥в кат≥он≥в ≤≤≤ анал≥тичноњ групи додати по 1-2 крапли розчину натр≥й карбонату Na2CO3 або кал≥й карбонату 2CO3. —постер≥гати по€ву кристал≥чних осад≥в ≥ зазначити њх кол≥р.
ќсади карбонат≥в розчин€ютьс€ в хлоридн≥й, н≥тратн≥й та ацетатн≥й кислотах.
BaCO3↓ + 2HNO3 → Ba(NO3)2 + H2O + CO2↑
BaCO3↓ + 2H+ F Ba2+ + H2O + CO2↑
BaCO3↓ + 2CH3COOH → Ba(CH3COO)2 + H2O + CO2↑
BaCO3↓ + 2CH3COOH F Ba2+ + 2CH3COO- + H2O + CO2↑
¬иконанн€ досл≥ду: до утворених осад≥в додати дек≥лька крапель н≥тратноњ або ацетатноњ кислоти. —постер≥гати розчиненн€ осад≥в.
3. ƒ≥€ хромат≥в
ат≥они ¬а2+ ≥ Sr2+ утворюють з розчинами K2CrO4 ≥ Na2CrO4 жовт≥ осади. альц≥й хромат —aCrO4 добре розчин€Їтьс€ у вод≥.
BaCl2 + 2K2CrO4 → BaCrO4↓ + 2KCl  = 1,2Ј10-10
= 1,2Ј10-10
Ba2+ + CrO  F BaCrO4↓
F BaCrO4↓
Sr(NO3)2 + K2CrO4 → SrCrO4↓ + 2KNO3  = 3,6Ј10-5
= 3,6Ј10-5
Sr2+ + CrO  F SrCrO4↓
F SrCrO4↓
¬иконанн€ досл≥ду:: в окрем≥ проб≥рки до 2-3 крапель розчин≥в кат≥он≥в ≤≤≤ анал≥тичноњ групи додати по 1-2 крапл≥ розчину кал≥й хромату K2CrO4. —постер≥гати по€ву осад≥в BaCrO4 i SrCrO4 та зазначити њх кол≥р.
–еакц≥њ Ѕар≥й-кат≥она
1. ƒ≥€ дихромат-≥ону
ѕри взаЇмод≥њ солей Ѕар≥ю з кал≥й дихроматом 2—r2ќ7 (в присутност≥ ацетат Ц ≥он≥в, €к≥ зм≥щують х≥м≥чну р≥вновагу в б≥к переб≥гу пр€моњ реакц≥њ) утворюЇтьс€ осад бар≥й хромату ¬а—rO4 жовтого кольору:
2¬а—l2 + 2—r2ќ7 + Ќ2ќ → 2¬а—rO4 ↓+ 2 —l + 2Ќ—l
2¬а2+ + —r2ќ  +Ќ2ќ F 2BaCrO4↓ + 2H+
+Ќ2ќ F 2BaCrO4↓ + 2H+
2H+ + 2CH3COќЦ F 2CH3CќOH
≤они Sr2+ ≥ —а2+ з дихромат-≥онами осад≥в не утворюють.
¬иконанн€ досл≥ду: ¬ проб≥рку до 2-3 крапель розчину сол≥ Ѕар≥ю додати 2-3 крапл≥ розчину кал≥й дихромату 2—r2ќ7 та 2 крапл≥ натр≥й ацетату CH3COќNa. —постер≥гати по€ву осаду жовтого кольору.
2. –еакц≥€ забарвленн€ полумТ€ (фармакопейна реакц≥€)
—ол≥ бар≥ю забарвлюють полум'€ пальника в жовто-зелений кол≥р.
¬иконанн€ досл≥ду: очищеною ≥ розжареною н≥хромовою дротинкою захопити с≥ль бар≥ю ≥ внести у безбарвну частину полумТ€ пальника. —постер≥гати зм≥ну забарвленн€ полум′€ у жовто-зелений кол≥р.
–еакц≥њ альц≥й-кат≥она
1. ƒ≥€ розчину сульфатноњ кислоти H2SO4
альц≥й-кат≥ониз сульфатною кислотою H2SO4 утворюють характерн≥ кристали г≥псу CaSO4 Ј2Ќ2ќ. ѕ≥д м≥кроскопом ц≥ кристали р≥зко в≥др≥зн€ютьс€ в≥д др≥бних кристал≥в BaSO4 ≥ SrSO4. ÷€ реакц≥€ м≥крокристалоскоп≥чна, вона дозвол€Ї ви€вити ≥они —а2+ в присутност≥ ≥он≥в ¬а2+ ≥ Sr2+.
CaCl2 + H2SO4 → CaSO4↓+ 2HCl
—а2+ + SO  F CaSO4↓
F CaSO4↓
¬иконанн€ досл≥ду: на предметне скло нанести 1 краплю розчину сол≥ альц≥ю ≥ 1 краплю концентрованоњ сульфатноњ кислоти H2SO4, упарити на вод€н≥й бан≥. –оздивитис€ кристали п≥д м≥кроскопом.
2. ƒ≥€ розчину амон≥й оксалату (NH4)2C2O4 (фармакопейна реакц≥€)
≤они —а2+ утворюють з оксалат-≥онами C2O42- б≥лий кристал≥чний осад.
—а—12 + (NH4)2C2O4 → CaC2O4↓+ 2NH4Cl
Ca2+ + C2O42- F —а—2ќ4↓
ќсад CaC2O4 розчин€Їтьс€ в м≥неральних кислотах, але не розчин€Їтьс€ в гар€ч≥й ацетатн≥й кислот≥ (на в≥дм≥ну в≥д бар≥й оксалату ¬aC2O4 та стронц≥й оксалату SrC2O4). ƒ‘” використовуЇ цю реакц≥ю дл€ визначенн€ гран≥чного вм≥сту дом≥шок —а2+-≥он≥в у препаратах.
¬иконанн€ досл≥ду: до 2-3 крапель розчину сол≥ альц≥ю додати 2-3 крапл≥ розчину амон≥й оксалату (NH4)2C2O4. —постер≥гати по€ву осаду б≥лого кольору.
3. ƒ≥€ розчину гл≥оксальг≥дрокс≥ан≥лу (фармакопейна реакц≥€)
–озчин гл≥оксальг≥дрокс≥ан≥лу в присутност≥ натр≥й г≥дроксиду, натр≥й карбонату ≥ хлороформу забарвлюЇ хлороформний шар у червоний кол≥р:
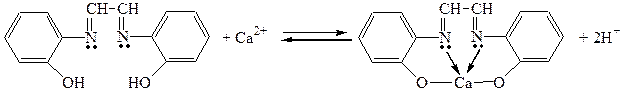
¬иконанн€ досл≥ду: до 2-3 крапель розчину сол≥ альц≥ю додати 3-4 крапл≥ натр≥й г≥дроксиду, 2-3 крапл≥ розчину гл≥оксальг≥дрокс≥ан≥лу та 2-3 крапл≥ хлороформу. «бовтати. —постер≥гати зм≥ну забарвленн€ хлороформного шару у червоний кол≥р.
4. ƒ≥€ розчину кал≥й гексац≥аноферату(≤≤) K4[Fe(CN)6] (фармакопейна реакц≥€)
≤они —а2+ з розчином K4[Fe(CN)6] у присутност≥ амон≥й хлориду NH4Cl утворюють б≥лий кристал≥чний осад амон≥йкальц≥й гексац≥аноферату(≤≤) (NH4)2Ca[Fe(CN)6].
CaCl2 + 2NH4Cl + K4[Fe(CN)6] → (NH4)2Ca[Fe(CN)6]↓ + 4KCl
Ca2+ + 2NH4+ + [Fe(CN)6]4- F (NH4)2Ca[Fe(CN)6]↓
ќсад (NH4)2Ca[Fe(CN)6] розчин€Їтьс€ в сильних кислотах але не розчин€Їтьс€ у розчин≥ ацетатноњ кислоти.
¬иконанн€ досл≥ду: до 2-3 крапель розчину сол≥ альц≥ю додати 2-3 крапл≥ розчину ацетатноњ кислоти, 2-3 крапл≥ розчину кал≥й гексац≥аноферату(≤≤) K4[Fe(CN)6] та дек≥лька кристал≥в амон≥й хлориду NH4Cl. —постер≥гати по€ву кристал≥чного осаду б≥лого кольору.
5. –еакц≥€ забарвленн€ полумТ€ (фармакопейна реакц≥€)
—ол≥ альц≥ю забарвлюють безбарвне полум'€ пальника в оранжево-червоний кол≥р.
¬иконанн€ досл≥ду: очищеною ≥ розжареною н≥хромовою дротинкою захопити с≥ль альц≥ю ≥ внести у безбарвну частину полумТ€ пальника. —постер≥гати зм≥ну забарвленн€ полумТ€ в оранжево-червоний кол≥р.
–еакц≥њ —тронц≥й-кат≥она
1. –еакц≥€ з г≥псовою водою
¬ насиченому розчин≥ г≥псу CaSO4 Ј2H2O концентрац≥€ ≥он≥в SO42Ц достатн€ дл€ випад≥нн€ осаду SrSO4.
Sr2+ + SO42Ц F SrSO4↓
јле внасл≥док утворенн€ пересиченного розчину, осад з Т €вл€Їтьс€ не одразу. ѕо€ву осаду прискорюють нагр≥ванн€м розчину. ƒану реакц≥ю використовують дл€ в≥дкритт€ Sr2+ - ≥он≥в, але т≥льки п≥сл€ в≥докремленн€ Ba2+ - ≥он≥в, оск≥льки
 (BaSO4) = 1,1Ј10-10 <
(BaSO4) = 1,1Ј10-10 <  (SrSO4) = 3,2Ј10-7
(SrSO4) = 3,2Ј10-7
¬иконанн€ досл≥ду: до 2-3 крапель розчину сол≥ —тронц≥ю додати 3-4 крапл≥ г≥псовоњ води (насиченого розчину кальц≥й сульфату) –озчин п≥д≥гр≥ти. —постер≥гати по€ву б≥лоњ каламут≥.
2. –еакц≥€ забарвленн€ полум'€
—ол≥ —тронц≥ю забарвлюють безбарвне полум'€ пальника у криваво-червоний кол≥р.
¬иконанн€ досл≥ду: очищеною ≥ розжареною н≥хромовою дротинкою захопити с≥ль —тронц≥ю ≥ внести у безбарвну частину полумТ€ пальника. —постер≥гати зм≥ну забарвленн€ полумТ€ в червоний кол≥р.
онтрольн≥ запитанн€
1. Ќазв≥ть груповий реагент III анал≥тичноњ групи. ¬каж≥ть умови осадженн€ кат≥он≥в ц≥Їњ групи.
2. ѕокаж≥ть, в €к≥й посл≥довност≥ будуть осаджуватис€ сульфати кат≥он≥в III анал≥тичноњ групи при д≥њ групового реагенту. ¬≥дпов≥дь обірунтуйте.
3. ѕокаж≥ть, €к зб≥льшити повноту осадженн€ CaSO4 при д≥њ групового реагенту.
4. «апиш≥ть реакц≥њ, за допомогою €ких ви€вл€ють у розчин≥ ≥они ¬а2+; Sr2+;—а2+.
5. ”каж≥ть, в €к≥ кольори забарвлюють полум'€ кат≥они ≤≤≤ анал≥тичноњ групи.
Ѕ≥олог≥чна роль та практичне використанн€ кат≥он≥в III анал≥тичноњ групи
та њх сполук у медицин≥ та фармац≥њ
альц≥й м≥ститьс€ в кожн≥й кл≥тин≥ т≥ла людини, його загальна масова частка в орган≥зм≥ складаЇ 1,4%. ƒобова потреба орган≥зму у альц≥њ 0,8-0,9 г. ¬ орган≥зм альц≥й потрапл€Ї, в основному, з харчовими продуктами (молоко, овоч≥, злаки). онцентрац≥€ ≥он≥в альц≥ю в орган≥зм≥ регулюЇтьс€ гормонами паращитовидних залоз. альц≥й Ї головним компонентом к≥стковоњ тканини ≥ зуб≥в, куди в≥н входить у вигл€д≥ солей —а—O3 та —а3(–ќ4)2. ≥льк≥сть кальц≥ю в к≥стках знаходитьс€ в залежност≥ в≥д на€вност≥ солей фосфатноњ кислоти. ¬≥д к≥лькост≥ альц≥ю в скелет≥ залежить його тверд≥сть, р≥ст ≥ м≥нерал≥зац≥€ к≥стки.
≤они альц≥ю беруть участь у передач≥ нервових ≥мпульс≥в, скороченн≥ м'€з≥в, регулюванн≥ роботи серц€, згортанн≥ кров≥. ≤они альц≥ю впливають на кислотно-лужну р≥вновагу, функц≥ю ендокринних залоз, мають протизапальну та десинсиб≥л≥зуючу д≥ю. ≤они альц≥ю знаход€тьс€ в б≥олог≥чному антагон≥зм≥ з ≥онами ал≥ю, Ќатр≥ю ≥ ћагн≥ю.
¬ сучасн≥й фармац≥њ нараховуЇтьс€ близько двадц€ти л≥карських препарат≥в, €к≥ м≥ст€ть кат≥он альц≥ю. јн≥они цих сполук складають залишки орган≥чних ≥ неорган≥чних кислот.
Ќайб≥льш широке використанн€ маЇ кальц≥й хлоридCaCl2, €кий зменшуЇ прониклив≥сть судин, маЇ протиалерг≥йну ≥ протизапальну д≥ю. …ого застосовують при алерг≥йних захворюванн€х (кропив'€нка), променев≥й хвороб≥, ревматизм≥, кровотечах, переломах к≥сток, шк≥рних захворюванн€х, а також при отруЇнн€х сол€ми ћагн≥ю, щавлевою кислотою, свинцем, ртуттю, фосгеном.
альц≥й карбонат осаджений (крейда осаджена) —а—ќ3 маЇ антацидну й адсорбуючу д≥ю. …ого призначають усередину при захворюванн€х харчового каналу, зовн≥шньо застосовують у вигл€д≥ присипок ≥ дл€ виготовленн€ зубних порошк≥в.
альц≥й сульфат (палений г≥пс) CaSO4 ЈH2O застосовуЇтьс€ дл€ г≥псових пов'€зок, при переломах, в зубол≥кувальн≥й практиц≥, щоб отримати зл≥пки порожнини рота.
альц≥й г≥дроксидCa(ќЌ)2 входить до складу цементу Ђ альцем≥нї ≥ пасти Ђ альцем≥н-пастаї, €к≥ використовуютьс€ в стоматолог≥чн≥й практиц≥.
–озчинн≥ у вод≥ сполуки альц≥ю вживаютьс€ в медицин≥ дл€ електрофорезу.
—тронц≥й концентруЇтьс€ головним чином у к≥стках, частково зам≥нюЇ альц≥й. …ого загальна масова частка складаЇ 1Ј10-3 %. ¬≥н граЇ важливу роль в утворенн≥ та м≥цност≥ зубноњ емал≥, а також бере участь у процесах к≥сткоутворенн€. ѕри введенн≥ до орган≥зму рад≥оактивного —тронц≥ю вдалось встановити, що в≥н накопичуЇтьс€ там, де йде ≥нтенсивний остеогенез. ѕри лейкозах вм≥ст —тронц≥ю в плазм≥ кров≥ зменшуЇтьс€, а в еритроцитах зб≥льшуЇтьс€. ¬изначенн€ к≥лькост≥ —тронц≥ю в плазм≥ та еритроцитах використовуЇтьс€ дл€ д≥агностики ≥ прогнозуванн€ захворювань лейкозом.
Ѕар≥й концентруЇтьс€ у визначних в≥дд≥лах очей, наприклад, у с≥тк≥вц≥ ока його близько 1,5%, однак значенн€ його залишаЇтьс€ доки незрозум≥лим. «агальна масова частка Ѕар≥ю в орган≥зм≥ складаЇ 1Ј10-5%. ѕри лейкозах вм≥ст Ѕар≥ю в плазм≥ кров≥ та еритроцитах зб≥льшуЇтьс€. „ерез це к≥льк≥сне визначенн€ Ѕар≥ю може бути д≥агностичним тестом ≥ прогнозом при л≥куванн≥ лейкоз≥в.
” медицин≥ знаходить застосуванн€ бар≥й сульфат ¬аSќ4. ќск≥льки в≥н не розчин€Їтьс€ н≥ у вод≥, н≥ у розведен≥й сол€н≥й кислот≥, €ка м≥ститьс€ в шлунковому соку, а також сильно поглинаЇ рентген≥вськ≥ та гамма-пром≥н≥, його застосовують дл€ рентген≥вськоњ д≥агностики захворювань харчового каналу.
ѕри отруЇнн≥ сол€ми Ѕар≥ю усередину дають розчинн≥ сол≥ сульфатноњ кислоти (Na2SO4, MgSќ4) дл€ утворенн€ важкорозчинного бар≥й сульфату. ’≥м≥чно чистий бар≥й сульфат вживаЇтьс€ при л≥куванн≥ виразки шлунка та дванадц€типалоњ кишки.
Ѕар≥й г≥дроксид ¬а(ќЌ)2 €к катал≥затор входить до складу цементу ≥ паст €к≥ використовуютьс€ в стоматолог≥чн≥й практиц≥.
ЋјЅќ–ј“ќ–Ќј –ќЅќ“ј
–≈ј ÷≤ѓ ј“≤ќЌ≤¬ IV јЌјЋ≤“»„Ќќѓ √–”ѕ»
(ј13+, Sn2+, Sn(IV), As(≤≤≤), јs(V), Zn2+,—r3+)
ћета: ¬ивчити реакц≥њ кат≥он≥в IV анал≥тичноњ групи та умови њх виконанн€
«агальна характеристика
ƒо IV анал≥тичноњ групи в≥днос€тьс€ кат≥они р -елемент≥в: ј13+, Sn2+, Sn(IV), As(≤≤≤), јs(V) ≥ d -елемент≥в Zn2+ ≥ —r3+.
√руповим реагентом кат≥он≥в IV групи Ї 6ћ розчин ќЌ або NaOH, в присутност≥ 3 % розчину г≥дроген пероксиду Ќ2ќ2. ѕ≥д час д≥њ групового реагенту спочатку випадають осади г≥дроксид≥в, €к≥ розчин€ютьс€ в надлишку реагенту. ѕрисутн≥сть Ќ2ќ2 спри€Ї утворенню г≥дроксо- та оксоан≥он≥в цих елемент≥в у найвищих ступен€х окисненн€.
√≥дроксиди кат≥он≥в IV анал≥тичноњ групи мають амфотерн≥ властивост≥, тому при д≥њ надлишку лугу вони розчин€ютьс€.
«агальноанал≥тичн≥ реакц≥њ кат≥он≥в IV анал≥тичноњ групи
1.ƒ≥€ 6ћ розчину лугу (д≥€ групового реагенту)
ат≥они IV анал≥тичноњ групи з розчинами натр≥й або кал≥й г≥дроксид≥в утворюють осади в≥дпов≥дних г≥дроксид≥в:
SnCl2 + 2NaOH → Sn(OH)2¯ + 2NaCl
Sn2+ + 2OH- F Sn(OH)2↓
Na2[SnCl6] + 4NaOH → Sn(OH)4↓ + 6NaCl
[SnCl6]2- + 4OH- F Sn(OH)4↓ + 6—l-
CrCl3+ 3NaOH → Cr(OH)3↓ + 3NaCl
Cr3+ + 3OH- F Cr(OH)3↓
ZnCl2 + 2NaOH → Zn(OH)2↓ + 2NaCl
Zn2+ + 2OH- F Zn(OH)2↓
јl—l3 + 3NaOH → Al(OH)3↓ + 3NaCl
A13+ + 3ќЌ- F Al(OH)3↓
¬иконанн€ досл≥ду: в окрем≥ проб≥рки до 2-3 крапель розчин≥в кат≥он≥в IV анал≥тичноњ групи додати 1-2 крапл≥ розчину лугу ќЌ або NaOH. —постер≥гати по€ву та зазначити њх кол≥р.
√≥дроксиди IV анал≥тичноњ групи мають амфотерн≥ властивост≥, тому розчин€ютьс€ €к у кислот≥ ( а ), так ≥ в надлишку лугу ( б ).
а) у кислот≥:
Sn(OH)2↓ + 2Ќ—l → SnCl2 + 2Ќ2ќ
Sn(OH)2↓+ 2Ќ+ F Sn2+ + 2Ќ2ќ
Sn(OH)4↓ + 6Ќ—l → Ќ2[Sn—l6] + 4Ќ2ќ
Sn(OH)4↓+ 4H+ + 6—l- F [SnCl6]2- + 4Ќ2ќ
—r(ќЌ)3↓ + 3Ќ—l → —r—l3 + 3Ќ2ќ
—r(ќЌ)3↓ + 3H+ F —r3+ + 3Ќ2ќ
Zn(OH)2↓ + 2Ќ—l → ZnCl2 + 2Ќ2ќ
Zn(OH)2↓ + 2H+ F Zn2+ + 2Ќ2ќ
јl(ќЌ)3↓ + 3Ќ—l → јl—l3 + 3Ќ2ќ
јl(ќЌ)3↓ + 3H+ F ј13+ + 3Ќ2O
б) у надлишку лугу:
Sn(OH)2↓ + 2NaOH → Na2[Sn(OH)4]
Sn(OH)2↓+ 2ќЌ- F [Sn(OH)4]2-
Sn(OH)4↓ + 2NaOH → Na2[Sn(OH)6]
Sn(OH)4↓ + 2OH- F [Sn(OH)6]2-
Cr(OH)3↓ + 3NaOH → Na3[Cr(OH)6]
Cr(OH)3↓ + 3ќЌ- F [Cr(OH)6]3-
Zn(OH)2↓ + 2NaOH → Na2[Zn(OH)4]
Zn(OH)2↓ + 2OH- F [Zn(OH)4]2-
Al(OH)3↓ + 3NaOH → Na3[Al(OH)6]
Al(OH)3↓ + 3ќЌ- F [Al(OH)6]3-
јрсен €к елемент поб≥чноњ п≥дгрупи V групи пер≥одичноњ системи маЇ ч≥тко виражен≥ неметал






