Сильные кислоты диссоциируют практически полностью и необратимо:
НСl → Н+ + Сl- или H2SO4 → 2H+ + SO42-
Расчет pH в растворах сильных кислот:
1) для одноосновных кислот
[Н+] = Скислоты; рН = -lg[H+] = -lg C;
2) для серной кислоты
[Н+] = 2 Скислоты; рН = -lg[H+] = -lg2 C.
Пример. Рассчитайте pH в 0,1М растворе НСl.
рН = -lg[H+] = -lg CHCl = -lg 0,1 = 1,0.
Диссоциация слабых кислот протекает обратимо. Сила кислоты характеризуется величиной константы кислотности (Ка) или ее отрицательным логарифмом (р Ка).
НА D Н+ + А-; Ка = [H+][A-]/[НА]; р Ка = -lg Ка.
Многоосновные кислоты диссоциируют ступенчато (исключение составляет серная кислота в разбавленном растворе). Так, в водном растворе трехосновной ортофосфорной кислоты наряду с молекулами Н3РO4 имеются ионы (в порядке уменьшения их количества) Н2РO4-, НРО42-, РО43-:

Расчет pH в растворах слабых кислот осуществляется следующим образом. Поскольку в результате диссоциации слабой кислоты НА D Н+ + А- в растворе образуется равное количество катионов и анионов [Н+] = [А-], а количество продиссоциировавших молекул пренебрежимо мало по сравнению с оставшимися неизменными [НА] = Скислоты - [Н+] ≈ Скислоты, следовательно
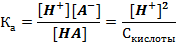
Отсюда
[Н+] =  .
.
После преобразования получаем
рН = ½ (p Ka – lg C).
При расчете pH растворов многоосновных кислот (кроме серной) в эту формулу подставляют только рКа1, так как диссоциация по второй и последующим ступеням практически не влияет на pH.
Сильные основания диссоциируют практически полностью и необратимо.
NaOH(ж) → Na+ + ОН-; Ва(ОН)2 → Ва2+ + 2ОН-
Расчет pH в растворах сильных оснований:
1) для однокислотных оснований
рОН = -lg[OH-] = -lg C; pH = 14 – рОН;
2) для двухкислотных оснований
рОН = -lg[OH-] = -lg2 C; pH = 14 – рОН.
Диссоциация слабых оснований протекает обратимо. Сила основания характеризуется величиной константы основности (Кв) или ее отрицательным логарифмом (p К b). В практике чаще используют константы кислотности кислоты, сопряженной данному основанию (K(BH+)), или ее отрицательный логарифм (рК(BH+)).
В + Н2O D ВН+ + ОН-; р К b = -lg К b,
ВН+ D В + Н+ p K (BH+) = -lg К (ВН+).
В результате преобразования получаем
р К b = 14 – p K (BH+).
Слабые многокислотные основания диссоциируют ступенчато. Аналогично кислотам каждая последующая ступень характеризуется меньшей степенью диссоциации и меньшей константой основности К b.
Fe(OH)3 D Fe(OH)2+ + ОН- α1, К b1;
Fe(OH)2+ D Fe(OH)2+ + ОН- α2, К b2;
Fe(OH)2+ D Fe3+ + ОН- α3, К b3;
α1 >> α2 >> α3; К b 1 >> К b 2 >> К b 3.
Расчет pH в растворах слабых оснований осуществляется следующим образом. В растворе однокислотного слабого основания В с концентрацией С [ОН-] = [ВН+]
[В] = C - [ВН+] ≈ C;
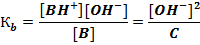
Отсюда
[OН-] = 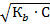 .
.
После преобразования получаем
рOН = ½ (p K b – lg C B);
pH = 14 – pOH = 14 – ½(p Kb – lg CB).
pH = ½(14 + p K (BH+) + lg CB)
Для многокислотных оснований расчет pH не имеет смысла, так как все они практически нерастворимы в воде.
Амфотерные электролиты (амфолиты) — электролиты, при диссоциации которых образуются и катионы водорода, и гидроксид-ионы. К ним относятся Н2O, Zn(OH)2, Аl(ОН)3, Сr(ОН)3 и ряд других.
Диссоциация амфотерного Zn(OH)2 протекает в соответствии с уравнением
2OН- + Zn2+ + 2Н2O D Zn(OH)2 + 2Н2О или при избытке щелочи [Zn(OH)4]2- + 2Н+
Соли. Практически все соли являются сильными электролитами (исключение составляют, например, соли Сu1+ и Hg+1).
Средние соли — электролиты, при диссоциации которых образуются катионы металлов или катион аммония (NH4+) и анионы кислотных остатков:
(NH4)2SO4 D 2NH4+ + SO42-; Na3PO4 D 3Na+ + PO43-.
Кислые и основные соли диссоциируют ступенчато. У кислых солей вначале отщепляются ионы металлов, а затем катионы водорода:
KHSO3 D К+ + HSO3- — первая ступень диссоциации (необратимая);
HSO3- D Н+ + SO32- — вторая ступень диссоциации (обратимая).
У основных солей вначале отщепляются кислотные остатки, а затем гидроксид-ионы:
Mg(OH)Cl D Mg(OH)+ + Сl- — первая ступень (необратимая);
Mg(OH)+ D Mg2+ + ОН- — вторая ступень (обратимая).
РЕАКЦИЯ НЕЙТРАЛИЗАЦИИ
Из протолитической теории Брёнстеда — Лоури следует, что чем сильнее основание, тем слабее сопряженная с ним кислота, и наоборот. Рассмотрим этот вывод на примере реакции нейтрализации.
Реакция нейтрализации — это реакция, протекающая между растворами кислоты и основания. Рассмотрим процесс нейтрализации слабой кислоты слабым основанием:
CH3COOH + NH3 D CH3COO- + NH4+
Количественной характеристикой этого процесса является константа нейтрализации

Умножив и числитель, и знаменатель дроби на [Н+], получим

Таким образом, глубина протекания реакции нейтрализации определяется соотношением по силе исходной и образующейся кислот.
Нейтрализация сильной кислоты сильным основанием выражается уравнением
Н+ + ОН- D Н2O
и представляет собой реакцию, обратную самоионизации воды. Поскольку в стандартных условиях K(H2O) равна 1,0 10-14, то константа нейтрализации равна
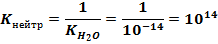
Таким образом, в стандартных условиях реакции между сильными кислотами и основаниями можно считать практически необратимыми.
РЕАКЦИИ ГИДРОЛИЗА СОЛЕЙ
Гидролиз солей относится к обменным реакциям, в которых одним из реагентов является вода. Суть гидролиза заключается во взаимодействии аниона слабой кислоты или катиона слабого основания с молекулами воды. Отсюда следует, что гидролизу подвергаются не растворенные соли, а лишь определенные ионы, входящие в их состав. Эти ионы должны соответствовать или слабым кислотам, или слабым основаниям Брёнстеда.
Анионы сильных кислот и катионы сильных оснований в процессе гидролиза не участвуют.
В зависимости от природы катиона и аниона средние соли можно разделить по отношению к гидролизу на четыре группы.
1. Соли, образованные анионом сильной кислоты и катионом сильного основания, например NaCl, K2SО4, в растворе диссоциируют полностью и гидролизу не подвергаются, и единственным протолитическим равновесием в таком растворе является ионизация воды:
Н2О(ж) D Н+ + ОН-
Среда в растворах таких солей нейтральная, pH = 7.
2. Соли, образованные анионом сильной кислоты и катионом слабого основания (NH4Cl, CuSО4), гидролизуются по катиону. Например, в растворе NH4Cl имеют место следующие протолитические равновесия:
NH4+ + Н2O D NH3 • Н2O + Н+
Н2О(ж) D Н+ + ОН-
Причем второе равновесие значительно сдвинуто влево. Среда в растворах таких солей кислая, pH < 7.
3. Соли, образованные анионом слабой кислоты и катионом сильного основания (NaF, K2SO3), гидролизуются по аниону. Например, в растворе NaF существуют следующие протолитические равновесия
F- + Н2O D HF + ОН-
Н2О(ж) D Н+ + ОН-
Опять же второе равновесие значительно сдвинуто влево. Среда в растворах таких солей щелочная, pH > 7.
В растворах кислых солей многоосновных кислот (NaHCO3, КН2РO4; К2НРO4 и т.п.) число протолитических равновесий увеличивается. Например, в растворе NaHCO3 имеют место следующие протолитические равновесия
НСО3- + Н2О D Н2СО3 + ОН-
НСО3- D СО32- + Н+
Н2О(ж) D Н+ + ОН-
Кислотность среды в таких случаях зависит от положения первых двух равновесий, и без знания значений соответствующих констант кислотности (рКа) многоосновной кислоты и результатов расчета pH невозможно определить, кислая среда или щелочная.
4. Соли, образованные анионом слабой кислоты и катионом слабого основания, например CH3COONH4, (NH4)2SO3, гидролизуются и по катиону и по аниону. Например, для CH3COONH4 в растворе можно записать следующие протолитические равновесия:
СН3СОО- + Н2О D СН3СООН + ОН-
NH4+ + Н2О D NH3 • Н2О + Н+
Н2О(ж) D Н+ + ОН-
В случаях, когда в результате гидролиза соли выпадает осадок или выделяется газ, гидролиз становится необратимым:
Al2S3 + 6Н2O D 2Аl(ОН)3↓ + 3H2S↑
В случаях расчета pH в растворах солей слабых кислот и слабых оснований кислая или основная реакция среды определяется относительной силой соответствующих кислот и оснований.
Процессы гидролиза оказывают непосредственное влияние на протекание многих реакций в организме. С ними связано постоянство кислотности крови и других жидкостей. Учет гидролиза необходим при решении вопроса о совместимости лекарственных препаратов, назначаемых пациенту, а также при попадании токсических веществ в организм для правильного оказания медицинской помощи.






