Примеры решения задач
Задача 1. Потенциал кадмиевого электрода, у которого токопроводящей фазой является насыщенный водный раствор Cd(OH)2, равен при температуре 298,15 K −0,55 В. Вычислите значение  при этой температуре.
при этой температуре.
Решение. Используя адаптированную для Т = 298,15 K форму уравнения Нернста для этого электрода

Поскольку Cd(OH)2 является малорастворимым соединением, то его насыщенный водный раствор является предельно разбавленным, поэтому в приведенном уравнении вместо активности используется равновесная концентрация ионов Cd2+.
После преобразования и подстановки данных находят
lg 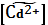 =
=  = −4,98.
= −4,98.
Откуда
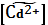 = 1,04∙10−5; [Cd2+] = 1,04∙10−5 моль/л/
= 1,04∙10−5; [Cd2+] = 1,04∙10−5 моль/л/
В насыщенном водном растворе Cd(OH)2 устанавливается равновесие, описываемое следующим уравнением
Cd(OH)2 (т) ⇄ Cd2+(р) + 2ОН−(р).
Видно, что равновесная концентрация ОН—ионов в 2 раза больше таковой ионов Cd2+ и таким образом составляет: 1,04∙10−5 ∙ 2 = 2,08∙10−5 моль/л.
По определению ПР этого соединения есть
 = [Cd2+]∙[OH−]2.
= [Cd2+]∙[OH−]2.
После подстановки данных в последнее выражение получают
 = 1,04∙10−5 ∙ (2,08∙10−5)2 = 4,50∙10−15 (моль/л)3.
= 1,04∙10−5 ∙ (2,08∙10−5)2 = 4,50∙10−15 (моль/л)3.
Табличное значение  = 4,30∙10−15 (моль/л)3.
= 4,30∙10−15 (моль/л)3.
Задача 2. Уравнениями опишите электродные процессы, протекающие в гальваническом элементе, схема которого:
(−) Ag | Pb | Pb2+ || Cu2+ | Cu | Ag (+).
Покажет ли ток амперметр, включенный во внешнюю цепь этого элемента, если  = 10−2,
= 10−2,  = 10−4? Приведите уравнение токообразующей реакции гальванического элемента и рассчитайте значение стандартной константы равновесия последней, а также величину совершаемой в нем электрической работы.
= 10−4? Приведите уравнение токообразующей реакции гальванического элемента и рассчитайте значение стандартной константы равновесия последней, а также величину совершаемой в нем электрической работы.
Решение. Данный гальванический элемент состоит из двух металлических электродов: свинцового и медного. Известно, что катодом в гальваническом элементе является электрод с большим потенциалом. Так как разница в значениях стандартных потенциалов этих электродов
Е 0 =  −
−  = 0,337 – (−0,126) = 0,463 > 0,3,
= 0,337 – (−0,126) = 0,463 > 0,3,
то по значению уже стандартных потенциалов можно однозначно сказать, что медный электрод – катод, а свинцовый – анод. В соответствии с этим уравнения электродных процессов таковы:
(−)А: Pb → Pb2+ + 2ē
(+)K: Cu2+ + 2ē → Cu.
Далее по адаптированной для Т = 298,15 K форме уравнения Нернста рассчитываю потенциалы свинцового и медного электродов
 = −0,126 +
= −0,126 +  lg10−2 = −0,185 В
lg10−2 = −0,185 В
 = 0,337 +
= 0,337 +  lg10−4 = 0,219 В.
lg10−4 = 0,219 В.
Так как ЭДС этого гальванического элемента
Е =  −
−  = 0,219 – (−0,185) = 0,404 ≠ 0
= 0,219 – (−0,185) = 0,404 ≠ 0
то, следовательно, ток во внешней цепи присутствует. Его наличие амперметр покажет отклонением стрелки.
Складывая уравнения анодного и катодного процессов, получают уравнение токообразующей реакции в ионно-молекулярной форме
Pb + Cu2+ ⇄ Pb2+ + Cu.
Предварительного подведения баланса электронов с помощью коэффициентов при этом не требуется, так как в анодном и катодном процессах их участвует одинаковое число (2). Значение стандартной константы равновесия K ° токообразующей реакции рассчитывают по стандартному уравнению изотермы
∆r G 0298= −R∙298,15∙2,3∙lg K °,
в котором
∆r G 0298= − nFE 0.
Подставляя в эти два уравнения значения постоянных F и R и затем их сочетая, получают
lg K ° = 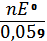 =
=  = 15,69,
= 15,69,
а
K ° = 4,90∙1015.
Столь большое значение последней говорит о сильном смещении положения равновесия в токообразующей реакции вправо, т.е. о практически необратимом ее протекании в направлении продуктов.
Электрическая работа в гальваническом элементе совершается за счет убыли энергии Гиббса, т.е.
A = −∆r G = nFE = 2∙96485,3∙0,404 = 77960,12 Дж.
Задача 3. Электролизу подвергают водный раствор, содержащий смесь солей: ZnBr2 и Cu(NO3)2. Электроды (анод и катод) являются графитовыми. Уравнениями описать протекающие при этом электродные процессы, а также привести суммарное уравнение электролиза.
Решение. Приведенные хорошо растворимые соли в водном растворе находятся в полностью диссоциированном состоянии (сильные электролиты)
ZnBr2 → Zn2+ + 2Br−
Cu(NO3)2 → Cu2+ + 2NO3−.
Таким образом, в растворе присутствуют: два вида анионов (Br− и NO3−), два вида катионов (Zn2+ и Cu2+) и молекулы растворителя (Н2О). С участием перечисленных частиц и будут протекать электродные процессы. В анодном процессе могут участвовать молекулы Н2О и анионы Br− и NO3−, в катодном − молекулы Н2О и катионы Zn2+ и Cu2+.
Возможными анодными полуреакциями являются следующие:
 2Н2О → О2↑ + 4Н++4ē < 0,993 В (при рН > 4)
2Н2О → О2↑ + 4Н++4ē < 0,993 В (при рН > 4)
(+)А: 2Br− → Br2 + 2ē  = 1,087 В
= 1,087 В
NO3−-ионы не окисляются в водных растворах
Известно, что на аноде в первую очередь окисляется самый сильный восстановитель – восстановитель с наименьшим значением потенциала.
Несмотря на то что потенциал первой полуреакции меньше, но из-за значительного перенапряжения выделения кислорода, особенно при больших плотностях тока, на аноде осуществляется вторая полуреакция. При этом в прианодном пространстве накапливаются NO3—ионы.
Возможными катодными полуреакциями являются следующие:
 2Н2О +2ē → Н2↑ + 2ОН− < −0,236 В (при рН > 4)
2Н2О +2ē → Н2↑ + 2ОН− < −0,236 В (при рН > 4)
(−)K: Zn2+ + 2ē → Zn  = −0,763 В
= −0,763 В
Cu2+ + 2ē → Cu  = 0,337 В
= 0,337 В
Известно, что на катоде в первую очередь восстанавливается самый сильный окислитель – окислитель с наименьшим значением потенциала. Выделение водорода, также как и кислорода, протекает со значительным перенапряжением, но все равно наибольшее значение потенциала наблюдается у третьей полуреакции, поэтому она и является катодной. Следует отметить, что в прикатодном пространстве накапливаются ионы Zn2+.
Суммарное уравнение электролиза получают сложением анодной и катодной полуреакций
2Br− + Cu2+ → Br2 + Cu.
Предварительного подведения баланса участвующих электронов при этом не требуется, так как их число в обеих полуреакциях одинаково.
С учетом одновременного накопления нитрат-ионов в прианодном пространстве и ионов цинка в прикатодном пространстве итоговое уравнение процесса выглядит так:
ZnBr2 + Cu(NO3)2 → Br2 + Cu + Zn2+ + 2NO3−.
Задача 4. В течение какого времени следует пропускать при Т = 298,15 K ток силой 5 А через 1 л водного раствора соли NaCl, чтобы рН последнего стал равным 12. Рассчитать объемы выделяющихся при этом на электродах газов. Электроды (анод и катод) графитовые. Анодный и катодный коэффициенты выхода по току одинаковы и равняются 1.
Решение. Так как NaCl – соль образована катионом сильного основания и анионом сильной кислоты, то в водных растворах она практически не подвергается гидролизу, т.е. рН ее водного раствора равен 7 и [H+] = [OH−] = 10−7 моль/л. В последнем присутствуют три вида частиц: ионы Na+ и Cl− в результате ее полной диссоциации как сильного электролита и молекулы растворителя – воды Н2О. Из сравнения значений потенциалов:  = 1,360 В,
= 1,360 В,  = −2,714 В, = −0,413 В, = 0,816 В с учетом высокого перенапряжения выделения кислорода и водорода следует, что возможными процессами являются следующие (см. решение задачи 3):
= −2,714 В, = −0,413 В, = 0,816 В с учетом высокого перенапряжения выделения кислорода и водорода следует, что возможными процессами являются следующие (см. решение задачи 3):
(+) А: 2Cl− → Cl2↑ + 2ē
(−) K: 2Н2О +2ē → Н2↑ + 2ОН−.
Далее находят рОН конечного раствора и активность ионов ОН− в нем:
рОН = 14 – рН = 14 – 12 = 2
рОН = –lg 
 = 10−2;
= 10−2;  = 10−2 моль/л.
= 10−2 моль/л.
По соотношению a = Cγ рассчитывают концентрацию этих ионов.
 = 1,10∙10−2 моль/л,
= 1,10∙10−2 моль/л,
где  − коэффициент активности ионов ОН− (справочная величина).
− коэффициент активности ионов ОН− (справочная величина).
Число моль эквивалентов ОН−-ионов, образовавшихся в результате электролиза, равно
n экв ОН− = ( − 10−7)∙ V = (1,1∙10−2 − 10−7)∙1 = 1,1∙10−2 моль экв.
− 10−7)∙ V = (1,1∙10−2 − 10−7)∙1 = 1,1∙10−2 моль экв.
В соответствии с законом эквивалентов такое же число моль эквивалентов выделилось на электродах и газов: на аноде – хлора, на катоде – водорода. Зная при с.у. их эквивалентный объем, равный 12,225 л/ моль экв, находят выделившиеся их объемы
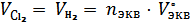 = 1,1∙10−2 ∙ 12,225 = 0,134 л.
= 1,1∙10−2 ∙ 12,225 = 0,134 л.
Подставляя полученное значение объема в объединенное выражение законов фарадея в виде
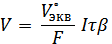
находят время электролиза
 = 212 с = 3,5 мин.
= 212 с = 3,5 мин.
Для нахождения последнего можно использовать и такую форму объединенного выражения законов Фарадея

 = 212 с.
= 212 с.






