Если раствор содержит в своем составе k компонентов, то молярная доля i -го вещества  в растворе равна отношению числа молей этого компонента
в растворе равна отношению числа молей этого компонента 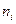 к общему (суммарному) числу молей всех веществ, составляющих раствор, т.е.
к общему (суммарному) числу молей всех веществ, составляющих раствор, т.е.
 (3.10)
(3.10)
Молярная доля – безразмерная величина, а сумма молярных долей всех компонентов раствора всегда равна единице: 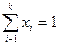 .
.
П р и м е р. Рассчитать молярные доли глюкозы С6Н12О6 и воды в 36%-м водном растворе глюкозы.
Р е ш е н и е. В 100г 36%-гораствора глюкозы содержится 36г глюкозы и 64г воды. Определим число молей глюкозы и воды:
 моль,
моль,  моль.
моль.
Раствор содержит два компонента (k =2) и  моль. Тогда молярные доли глюкозы и воды в растворе, соответственно, составляют:
моль. Тогда молярные доли глюкозы и воды в растворе, соответственно, составляют:
 и
и  .
.
Проверим вычисления: сумма молярных долей компонентов раствора
 .
.
3.3 Примеры решения типовых задач
3.3.1 Вычисление процентного содержания веществ в растворе
З а д а ч а 1. В 420г воды растворено 180г нитрата кальция Ca(NO3)2. Определить процентное содержание нитрата кальция в растворе.
Р е ш е н и е. Общая масса раствора составляет: 420+180=600г. Процентное содержание находим из пропорции:
180 г Ca(NO3)2 содержится в 600г раствора
x г Ca(NO3)2 содержится в 100г раствора
Тогда
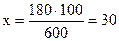 г, т.е. 30%-ый раствор.
г, т.е. 30%-ый раствор.
З а д а ч а 1. В 450г воды растворили 50г CuSO4×5H2O. Вычилить процентное содержание кристаллогидрата и безводной соли растворе.
Р е ш е н и е. Общая масса раствора составляет: 450+50=500г. Процентное содержание кристаллогидрата CuSO4×5H2O находим из пропорции: 50 г CuSO4×5H2O содержится в 500г раствора
x г CuSO4×5H2O содержится в 100г раствора
Тогда  г, т.е. 10% масс.
г, т.е. 10% масс.
Молярная масса кристаллогидрата составляет 63.5+32+4×16+5×18=249.5, а безводной соли 159.5 г×моль-1. Поэтому в 50г кристаллогидрата содержится 51.96г безводной соли. Следовательно, процентное содержание безводной соли CuSO4 составит
 % масс.
% масс.
3.3.2 Вычисление массы растворенного вещества или растворителя по данной массе раствора и его концентрации
З а д а ч а 1. Сколько граммов соли и воды содержится в 800г 12%-го раствора NaNO3?
Р е ш е н и е. Поскольку концентрация соли составляет 12%, можно составить пропорцию:
12г соли содержится в 100г раствора
x г содержится в 800 г раствора
Отсюда
 г NaNO3.
г NaNO3.
Тогда масса растворителя составит 800-96=704г.
3.3.3 Вычисление массы раствора определенной концентрации по данной массе растворенного вещества или растворителя
З а д а ч а 1. Сколько граммов 3%-го раствора сульфита магния MgSO4 можно приготовить из 100г MgSO4×7H2O?
Р е ш е н и е. Молярная масса кристаллогидрата составляет 24+32+4×16+7×18=246, а безводной соли 120 г×моль-1. Поэтому в 100г кристаллогидрата содержится 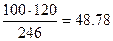 г безводной соли.
г безводной соли.
Поскольку раствор 3%-ый, можно составить пропорцию:
3г содержится в 100г раствора
48.78г содержится в x г раствора
Следовательно, можно приготовить
 г раствора.
г раствора.
З а д а ч а 2. Сколько граммов 5%-го раствора гидроксида калия можно приготовить растворением KOH в 100г воды?
Р е ш е н и е. В 5%-ом растворе 95г воды находится в 100г раствора. Если воды взять 100г, то можно приготовить  г раствора.
г раствора.
3.3.4 Вычисление массы растворенного вещества или массы растворителя, необходимой для получения раствора определенной концентрации
З а д а ч а 1. Сколько граммов HCl следует растворить в 250г воды для получения 10%-го раствора HCl?
Р е ш е н и е. В 10%-ом растворе 90 г воды и 10г кислоты, следовательно, если взять 250г воды, то потребуется x г кислоты
 г.
г.
З а д а ч а 2. В каком количестве граммов воды следует растворить 100г MgSO4×7H2O для получения раствора, содержащего 5% безводной соли?
Р е ш е н и е. В 100г кристаллогидрата содержится 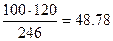 г безводной соли (см. задачу 2, раздел 1.3.3) и 51.22 г воды. Так как раствор 5%-ый, то на 5 г безводной соли нужно взять 95г воды. Следовательно, при растворении 48.78г соли потребуется
г безводной соли (см. задачу 2, раздел 1.3.3) и 51.22 г воды. Так как раствор 5%-ый, то на 5 г безводной соли нужно взять 95г воды. Следовательно, при растворении 48.78г соли потребуется  г воды. Таким образом, остается добавить 926.82 – 51.22=875.6г H2O.
г воды. Таким образом, остается добавить 926.82 – 51.22=875.6г H2O.
Можно сначала найти общую массу раствора, которая составит 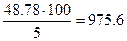 г, а затем массу растворителя 975.6 – 100 = 875.6г воды.
г, а затем массу растворителя 975.6 – 100 = 875.6г воды.
3.3.5 Смешение растворов разных концентраций
Задача 1. Сколько граммов 20%-го раствора гидроксида калия КОН надо прибавить к 250 г 90%-го раствора, чтобы получить 50%-й раствор КОН?
Решение. Задачи такого типа легко решаются при помощи «диагональной схемы» (или «правила креста»). Диагональная схема строится так: точкой пересечения двух отрезков прямой обозначают концентрацию смеси (50%). У концов обоих отрезков по одну сторону от точки пересечения указывают концентрации составных частей смеси (90 и 20%),а по другую – разности концентраций смеси и ее составных частей (90—50 = 40% и 50—20 = 30%). «Диагональная схема» для данной задачи имеет вид:

Количества исходных растворов, необходимые для приготовления смеси, обратно пропорциональны разностям между концентрациями заданного и менее концентрированного растворов и более концентрированного и заданного раствора. Массу 20%-го раствора КОН обозначим через х, тогда
 ,
,
следовательно, 3x =1000 и х=333,3 г. Таким образом, для получения 50%-го раствора КОН к 250 г 90%-го раствора надо прибавить 333.3 г 20%-го раствора КОН.
Задача 2. Сколько литров 6N раствора гидроксида натрия NaОН следует добавить к 4.5 л 0,8N раствора гидроксида калия КОН, чтобы смешанный раствор оказался двунормальным?
Решение. В 4,5 л 0.8N раствора КОН содержится 4.5×0.8 = 3.6 молей эквивалентов КОН. Если добавить х л 6N раствора КОН,.то общее число молей эквивалентов составит: 3.6 + 6 х. Общий объем в литрах составит 4.5 + х. По условию задачи, в каждом литре смешанного раствора должно содержаться 2 моля эквивалентов, т. е.:
 ; x =1.35л.
; x =1.35л.
3.3.6 Расчеты, связанные с использованием плотности растворов
Задача 1. Сколько граммов 10%-го раствора серной кислоты H2SO4 требуется для обменного взаимодействия со 100мл 13.7%-го раствора Na2CO3 (r=1.145 г×см-3)?
Р е ш е н и е. 100 мл раствора массой 114.5 г содержат
114.5×0.137=15.68г Na2CO3.
По уравнению реакции
Na2CO3 + H2SO4 = Na2SO4 +CO2 + H2O
находим необходимое количество серной кислоты, учитывая, что молярные массы Na2CO3 и H2SO4 соответственно равны 106 и 98 г×моль-1:
 г.
г.
10%-го раствора H2SO4 потребуется  г.
г.
Задача 2. Сколько миллилитров 35%-го раствора аммиака (р= 0,94 г×см-3) требуется для образования 33 г (NH4)2S04?
Решение. Молярные массы аммиака и сульфата аммония составляют 17 и 132 г×моль -1, соответственно.
Из уравнения реакции
2NH3 + H2SO4 = (NH4)2SO4
находим массу аммиака, необходимую для получения 33 г (NH4)2S04
 г.
г.
Следовательно, 35%-го раствора потребуется
 г или
г или  мл.
мл.
Задача 3. Сколько миллилитров 32,5%-го раствора NН3 (р = 0,888 г/см3) требуется для образования сульфата аммония (NН4)2S04 при взаимодействии с 250 мл 27,3%-го раствора серной кислоты Н2S04 (р = 1,2 г/см3)?
Решение. Масса раствора кислоты составляет 250×1,2 = 300г. Раствор имеет концентрацию 27.3%, следовательно, содержит  г серной кислоты. По уравнению реакции
г серной кислоты. По уравнению реакции
2NH3 + H2SO4 = (NH4)2SO4
вычисляем массу аммиака, необходимую для взаимодействия с этим количеством кислоты:  г. Поскольку раствор аммиака имеет концентрацию 32.5%, рассчитанное количество аммиака будет содержаться в
г. Поскольку раствор аммиака имеет концентрацию 32.5%, рассчитанное количество аммиака будет содержаться в
 г раствора. Этому количеству соответствует объем
г раствора. Этому количеству соответствует объем  мл.
мл.
3.3.7 Переход от одних способов выражения концентрации к другим
Задача 1. Определить молярную, эквивалентную и моляльную концентрацию сульфата меди (II) в 10%-м растворе СuS04 (р = 1.107 г/см3).
Решение. Определим молярную и эквивалентную массы СuS04:
 г×моль-1,
г×моль-1,  г×моль-1.
г×моль-1.
В 100 г 10%-го раствора сульфата меди содержится 10 г СuS04 и 90 г воды. Отсюда моляльная концентрация СuSO4 в данном растворе
 моль/кг H2O.
моль/кг H2O.
Молярная концентрация показывает количество моль СuSO4 в 1 л раствора. Нам же известно, что в 100г 10%-го раствора масса СuSO4 составляет  г. Объем 10%-го раствора массой
г. Объем 10%-го раствора массой  г
г
 см3.
см3.
Тогда молярная концентрация
 моль/л»0.7М.
моль/л»0.7М.
Эквивалентная концентрация характеризует количество молей эквивалентов сульфата меди (II), содержащееся в 1 л раствора.
1 моль СuSO4 содержит 2 моль эквивалентов сульфата меди. Значит,
 моль/л = 1.4 N.
моль/л = 1.4 N.
Задача 2. Определить эквивалентную и молярную концентрации 10%-го раствора Na2СО3, плотность которого  1.202 г/см3.
1.202 г/см3.
Решение. Определим молярную и эквивалентную массы Na2СО3:
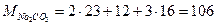 г×моль-1,
г×моль-1,  г×моль-1
г×моль-1
В 100 г 10%-го раствора масса Nа2С03 составляет  г, а объем 10%-го раствора массой 100 г равен
г, а объем 10%-го раствора массой 100 г равен  см3.
см3.
Поэтому молярная концентрация этого раствора
 моль/л = 1.04М.
моль/л = 1.04М.
Поскольку 1 моль Na2С03 содержит 2 моль эквивалентов Na2С03, то
 моль/л = 2.08N.
моль/л = 2.08N.
3.3.8 Приготовление разбавленных растворов из концентрированных
Задача 1. Сколько литров 30%-го раствора азотной кислоты НN03
(р == 1.18 г/см3) требуется для приготовления 20 л 0.5 М раствора этой кислоты?
Решение. Используя формулу (1.8), определяем, сколько граммов азотной кислоты содержится в 20 л ее 0.5М раствора:
 ;
;  г.
г.
В 30%-ом растворе HNO3 на 100г раствора приходится 30г HNO3, поэтому 630г HNO3 будет приходиться на  г раствора азотной кислоты. Зная плотность, найдем объем этого раствора
г раствора азотной кислоты. Зная плотность, найдем объем этого раствора
 см3 = 1.78л
см3 = 1.78л
Следовательно, чтобы приготовить 20 л 0,5М раствора НNО3, надо израсходовать 1.78л 30%-го раствора этой кислоты.
Задача 2. Сколько миллилитров 9.5%-го раствора Nа2СО3  (р = 1,10 г/см3) следует добавить к 100 г воды для получения 3%-го раствора?
(р = 1,10 г/см3) следует добавить к 100 г воды для получения 3%-го раствора?
Решение. Обозначим искомый объем раствора через х мл. Масса его равна  г, а масса содержащегося в нем Nа2С03
г, а масса содержащегося в нем Nа2С03  г.
г.
По условию задачи, масса растворенного вещества составляет 3% от массы полученного раствора  г:
г:
 ,
,
Откуда  см3(мл).
см3(мл).
3.3.9 Отношения между эквивалентными концентрациями и объемами растворов реагирующих веществ
Задача 1. Сколько литров 0.1N раствора нитрата серебра необходимо для обменной реакции с 0.5 л 0.3N раствора хлорида алюминия?
Решение. Из закона эквивалентов следует, что


и  л раствора нитрата серебра.
л раствора нитрата серебра.
Задача 2. Определить нормальность раствора гидроксида калия КОН, если на нейтрализацию 0.035 л 0.3N раствора фосфорной кислоты H3PO4 израсходовано 0,02 л раствора КОН.
Решение. Из закона эквивалентов следует, что число молей эквивалентов всех участвующих в химической реакции веществ одинаково. В реакции участвует 0.035×0.03= 0.0105 моль эквивалентов H3PO4. Для нейтрализации фосфорной кислоты требуется такое же число моль эквивалентов щелочи, т.е.  и
и
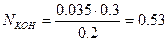 N
N
3.4 Многовариантные задания
3.4.1 Задание 1
Определить количество растворенного вещества А (в граммах и молях), которое содержится в g граммах водного раствора заданной концентрации (%масс.) (таблица 3.1).
Таблица 3.1 – Варианты задания 1
| № варианта | Вещество А | Масса раствора g, г | Концентрация, % масс |
| K2CO3 H2SO4 HCl KOH AgNO3 K2Cr2O7 KMnO4 KCl MnSO4 H4P2O7 NaClO4 NaCl HClO4 H2O2 Ba(NO3)2 KI NaOH H3BO3 HNO3 CH3COOH CuSO4 ZnSO4 CdSO4 KNO3 NaPO3 Na2SO3 Na2S2O3 Na2CO3 KOH NH4Cl | 8.0 20.0 15.0 17.5 3.5 10.0 5.0 10.0 7.0 6.0 5.0 12.0 7.5 1.0 2.0 6.0 5.0 1.0 25.0 78.0 25.0 33.0 25.0 20.0 17.5 45.0 34.0 15.0 8.0 3.0 |
3.4.2 Задание 2
Определить количество растворенного вещества А (в граммах и молях), которое содержится в объеме V раствора c плотностью r и заданной концентрацией (% масс.) (таблица 3.2).
Таблица 3.2 – Варианты задания 2
| № варианта | Вещество А | Объем раствора V, мл | Концентрация, % масс | Плотность раствора r, г/см3 |
| NH4OH AlCl3 NaOH CaCl3 Na2CO3 CH3COOH KOH HClO4 K2CO3 K2Cr2O7 AgNO3 CuSO4 ZnCl2 H3PO4 Na2SO4 KI Fe2(SO4)3 BaCl2 HNO3 CuSO4 FeCl3 Na2S2O3 CdSO4 HCl KBr ZnCl2 Al2(SO4)3 LiOH K2Cr2O7 NaBO3 | 20.0 8.0 50.0 40.0 4.0 8.0 5.1 67.0 50.0 8.0 20.0 4.0 60.0 35.5 8.0 50.0 4.0 4.0 27.0 8.0 50.0 40.0 4.0 13.5 40.0 8.0 20.0 4.0 4.0 20.0 | 0.923 1.071 1.525 1.395 1.039 1.010 1.045 1.635 1.540 1.055 1.194 1.040 1.568 1.220 1.072 1.545 1.033 1.084 1.160 1.084 1.551 1.382 1.547 1.065 1.374 1.071 1.226 1.043 1.026 1.220 |
3.4.3 Задание 3
Определить массу растворенного вещества А (в граммах), содержащуюся в водном растворе с молярной концентрацией  и объемом V (таблица 3.3).
и объемом V (таблица 3.3).
Таблица 3.3 – Варианты задания 3
| № варианта | Вещество А | Объем раствора V, мл | Молярная концентрация  , моль/л , моль/л
|
| HCl AlCl3 Sr(NO3)2 K2Cr2O7 LiOH NaHCO3 H3BO3 KClO3 Na2S CH3COOH Co(NO3)2 KBrO3 KCl HCl HNO3 NaClO4 (NH4)2CO3 Na2SO4 AlCl3 H2S Na2Cr2O7 SnCl2 NaH2PO4 MgSO4 CuBr2 KCN NH4CNS AgNO3 HCl AlCl3 | 0.15 0.30 0.25 0.18 0.31 0.20 0.10 0.31 0.15 0.50 1.00 0.50 0.20 7.00 11.00 1.50 2.00 0.17 0.20 0.08 0.12 0.14 0.17 0.15 0.20 1.50 0.25 0.15 0.20 0.35 |
3.4.4 Задание 4
Определить молярную концентрацию водного раствора вещества А, если задана (% масс.) концентрация и плотность раствора r (таблица 3.4).
Таблица 3.4 – Варианты задания 4
| № варианта | Вещество А | % масс. | Плотность r, г/см3 |
| NaOH CuSO4 CdSO4 BaCl2 HCl Na2S2O3 FeCl3 HNO3 Fe2(SO4)3 KI Na2SO4 H3PO4 ZnCl2 CuSO4 AgNO3 K2Cr2O7 K2CO3 HClO4 AlCl3 KBr H2SO4 H3PO4 HNO3 HClO4 LiNO3 KNO3 NaNO3 RbNO3 K2SO4 NaOH | 50.0 8.0 40.0 4.0 13.5 40.0 50.0 27.0 4.0 50.0 50.0 35.5 50.0 4.0 20.0 8.0 50.0 67.0 8.0 40.0 70.0 70.0 19.5 25.0 56.0 25.0 10.0 30.0 10.0 30.0 | 1.525 1.083 1.547 1.034 1.065 1.382 1.551 1.160 1.033 1.545 1.545 1.220 1.568 1.040 1.194 1.055 1.540 1.635 1.071 1.374 1.616 1.513 1.115 1.160 1.427 1.170 1.067 1.257 1.081 1.525 |
3.5 Контрольные вопросы
1. Какие системы называются раствором?
2. Как можно выразить количество вещества в растворе? Понятия моль и моль эквивалент.
3. На какие группы в зависимости от способа выражения можно разделить концентрации?
4. Какие способы выражения концентраций относятся к массовым концентрациям?
5. Что показывает процентная концентрация по массе? Приведите примеры.
6. Как выражается моляльная концентрация? Приведите примеры.
7. Какие способы выражения концентраций относятся к объемным концентрациям? Приведите примеры.
8. В каких случаях совпадают молярная и нормальная концентрации?
9. Приведите пример выражения безразмерной концентрации.
4 Общие рекомендации к лабораторному практикуму
В лабораторном практикуме бакалавр знакомится с методами физико-химических исследований и получает навыки самостоятельной экспериментальной работы. При прохождении практикума студент должен научиться пользоваться современной аппаратурой и приборами, освоить методы обработки результатов измерений, используя графические и аналитические методы, научиться оформлять результаты работы в виде наглядных цифровых и графических материалов.
Лабораторные занятия требуют строжайшей дисциплины, тщательного соблюдения и техники безопасности и правил работы в лаборатории.
Перед работой студент изучает теоретический материал [3], необходимый для выполнения лабораторной работы. К лабораторной работе допускаются студенты, сдавшие коллоквиум к работе в объеме рекомендованной литературы.
Студент заранее готовит бланк отчета и лист наблюдений. В листе наблюдений заготавливаются таблицы для записи измерений. В бланк отчета по лабораторной работе включают:
· наименование работы, фамилия студента, номер группы и дата работы;
· схему установки;
· расчетные формулы;
· таблицу измерений с указанием единиц измерения;
· обработку экспериментальных данных и расчет определяемой величины;
· график на миллиметровой бумаге (если он предусмотрен по содержанию работы).
Отчет считается принятым, если он содержит удовлетворительные
данные измерений и оформлен в соответствии с перечисленными требованиями. Студент выполняет индивидуально 3 работы из предложенного списка.
4.1 Темы лабораторных работ
1. Определение количества моль кислоты (щелочи) методом титрования с цветным индикатором.
2. Определение количества моль сильной кислоты методом потенциометрического титрования с водородным электродом.
3. Определение количества моль сильной (слабой) кислоты или смеси кислот методом кондуктометрического титрования.
4. Определение константы гидролиза соли.
5. Определение рН раствора с помощью стеклянного электрода.
В приложение приводятся образцы титульных листов отчетов.
Литература
а) основная
1 Глинка, Н. Л. Общая химия: учебное пособие для вузов / Н. Л. Глинка.– 16-е изд., испр. и доп. – М.: Изд-во Юрайт, 2010. - 896 с.
б) вспомогательная
1 Суворов, А. В. Общая химия: учебник для вузов / А. В. Суворов, А. Б. Никольский. - СПб.: Химиздат, 2007. - 624 с.
2 Практические работы по физической химии: учебное пособие для вузов / Ю. П. Акулова [и др.], под ред. К. П. Мищенко, А. А. Равделя, А. М. Пономаревой. - 5-е изд., перераб. - СПб.: Профессия, 2002. - 384 с.
3 Краткий справочник физико-химических величин / Под ред. А. А. Равделя, А. М. Пономаревой. - 11-е изд., испр. и доп. - М.: Аз-book, 2009. - 240 с.
4 Задачи и упражнения по общей химии /Под ред. Н. В. Коровина. - М.: Высшая школа, 2003. – 255 с.
5 Фролов, В. В. Химия: учебное пособие / В. В. Фролов. - М.: Высшая школа, 1986. - 543 с.
6 Коровин, Н. В. Общая химия: учебник для технических направлений и спец. вузов / Н. В. Коровин. - М.: Высшая школа, 2007. - 557 с.
7 Глинка, Н. Л. Задачи и упражнения по общей химии: учебное пособие для нехим. спец. вузов / Н. Л. Глинка. - М.: Интеграл-Пресс, 2006. - 265 с.
ПРИЛОЖЕНИЕ
(Обязательное)
Образцы титульных листов лабораторных работ

Государственное образовательное учреждение
Высшего профессионального образования
Санкт-Петербургский государственный технологический институт
(Технический университет)






